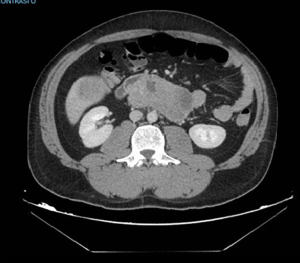
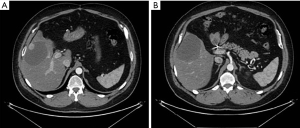
Voluminous abdominal gastrointestinal stromal tumor of unknown origin manifested with bleeding in a young man: synchronous management of the emergency and oncological approach—case report
Introduction
Gastrointestinal stromal tumor (GISTs) are rare tumors of the gastrointestinal tract, which cover about 1–2% of gastrointestinal neoplasms with an unadjusted incidence of around 1/100,000/year. They are also the most common non-epithelial neoplasms of the gastrointestinal tract and they are associated with a high rate of malignant transformation (1). These tumors have been characterized by practical problems for many years due to the lack of diagnostic criteria, partly also due to the incomplete understanding of its origin and differentiation mechanisms (2). Over the years, however, it has been discovered that their origin derives from the wall of the hollow viscera, from the esophagus to the anus (3).
They are more common in the stomach (40–60%) while a minor part repeatedly involves jejunum/ileus (25–30%), duodenum (5%), colorectal (5–15%) and esophagus (<1%) (4). There are also much rarer EGIST, which can involve retroperitoneum, mesentery, and omentum, without affecting the gastrointestinal tract. However, they have immunohistochemical and molecular characteristics similar to GISTs and for this reason, they are called this way (4,5). As regards the morphological aspect, the cellular morphology of GISTs falls into one of three relatively uniform categories: spindle cell type, epithelioid type, and mixed type (4). The immunohistochemical and molecular characteristics of GIST are: positivity for CD117, an antigen expressed by the C-kit mutation; and/or DOG1+, PKC-theta, PDGFRA+; CD34 (not specific for GIST) (4,6).
The clinical presentation depends on the primary localization of the neoplasm, however in 18% it is asymptomatic, and it is accidentally discovered during endoscopies, radiological examinations or surgical operations performed for other reasons, especially if it is small in size. More often, however, they are associated with non-specific symptoms such as early satiety, nausea or vomiting. A lower quota causes gastrointestinal bleeding, abdominal pain, mechanical jaundice, intestinal obstruction, massive intraperitoneal bleeding secondary to necrosis and/or ulceration of the neoformation. Gastrointestinal bleeding is the most common and the most dangerous complication, often necessitating emergency surgery. The causes of GISTs bleeding are similar to those of other primary gastrointestinal malignant tumors; however, the proportion of GISTs that bleed is greater (1,3,4).
Prognostic factors are the mitotic rate, tumor size and tumor site (gastric GISTs have a better prognosis than small bowel or rectal GISTs). Tumour rupture is an additional adverse prognostic factor. According to a consensual stratification of the risk group, it is possible to classify in 4 risk categories: a very low-risk group (<2 cm and <5 mitoses/50 HPF), a low-risk group (2–5 cm and <5 mitoses/50 HPF), an intermediate-risk group (<5 cm and 6–10 mitoses/50 HPF or 5–10 cm and <5 mitoses/50 HPF), and high-risk group (>5 cm and >5 mitoses/50 HPF or >10 cm regardless of mitotic activity) (7).
GISTs show a wide spectrum of radiological appearances depending on imaging technique and tumor size, site of origin and growth pattern (8). They can be identified on abdominal ultrasound, CT scan, magnetic resonance imaging (MRI), and positron-emission tomography (PET) (1).
Although abdominal ultrasound is often the first test performed on a patient with pain or an abdominal mass, the abdominal mass is often so a huge mass filling the abdomen, with necrosis that the organ of origin is not identifiable (9). Contrast-enhanced abdominal and pelvic CT scan is the investigation of choice for staging and follow-up.
MRI has a comparable diagnostic yield and lacks radiation exposure, and it could be used in a patient who cannot receive intravenous contrast. MRI can, furthermore, provide additional information on the tumor response to imatinib treatment, including high signal intensity on T2-w images and decrease of vascularized areas of GIST manifestations (4,10). MRI is preferred when identifying rectal GISTs, liver metastasis, hemorrhage, and necrosis of tumors. However, when compared to MRI, CT has the advantage of displaying the thickness of the entire small bowel, leading to better visualization of deep ileal loops and mesentery (1,6).
PET provides functional information that may help in staging, especially when combined with morphological information provided by CT (8).
Endoscopy has a limited role in the detection of GISTs due to the high prevalence of extraluminal tumors (1), and alone cannot accurately distinguish between intramural and extramural tumors. By contrast, endoscopic ultrasonography (EUS) has provided a major breakthrough for characterizing the masses and enabling guided-tissue acquisition for immunohistochemistry. A preoperative biopsy is not generally recommended for a resectable lesion with a high suspicion for GIST. However, a biopsy is preferred to confirm the diagnosis if metastatic disease is suspected or if preoperative imatinib is considered prior to attempted resection in a patient who has a large locally advanced lesion thought to represent a GIST (4).
In addition, radiological tests can also be used to evaluate the response to chemotherapy such as tumor size and tumour density on CT scan, or consistent changes in MRI or contrast-enhanced ultrasound. Even PET scan showed high sensitivity in the early evaluation of response to therapy and can be useful when evaluating the presence of early response when it is particularly useful (e.g., preoperative cytoreductive treatments) (6). We present the following case series in accordance with the reporting checklist (available at http://dx.doi.org/10.21037/acr-20-70).
Case presentation
The purpose of this case report is to describe our experience in managing a young patient who came to our attention for melena and anemia. On November 15, 2015, a 47-year-old patient came to the emergency room for the onset of melena ed hypotension. The hemoglobin values were 7.3 g/dL, stable vital signs. His remote pathological history was negative. He was admitted to the Gastroenterology department where he performed blood transfusion with 3 RBC units. Subsequently, he performed an EGDS, which was negative for neoformations up to the second duodenal portion.
The only evidence was the presence of pyloric mucosal erosions. No blood in the cavity or bleeding lesions were found. Considering the negativity of the endoscopic examination, he performed a CT scan that showed voluminous retroperitoneal thickening of 10 cm × 5 cm, poorly dissociable from the head of the pancreas and from the uncinate process, with poly-lobulated wall profiles and inhomogeneous contrast enhancement (Figures 1-3). The lesion infiltrated and subverted the third portion of the duodenum, creating a stenosis and poststenotic ectasia. The radiologist was unable to distinguish between pancreatic or duodenal primitiveness. The radiologist also described the presence of some adjacent partially colliquated lymphoadenomegalies and multiple liver replicative lesions, the largest one of 76 mm at the VI segment.
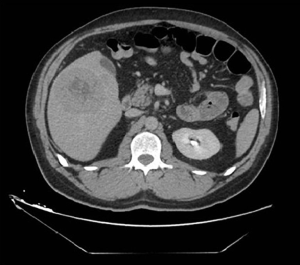
In addition, there were multiple bilateral nodular lung lesions of 1 cm suggestive of metastasis. A second EGDS was then performed with exploration also of the third portion of the duodenum, which showed the presence of a bleeding ulcer of 3 cm, covered with fibrin at the transition between II and III duodenal portion. Adrenaline infiltration was performed and bleeding was no longer visible at the end of the procedure.
The liver aspect was deepened by CEUS which described the known liver lesion characterized by lively arterial enhancement of the peripheral portion, followed by wash-out with portal-late hypoechogenicity. Smaller nodules and retroperitoneal neoformation showed similar contrasting behavior. Since the doubt could not be resolved between NET and angiosarcoma or GIST, a liver nodule biopsy was performed. The histological examination described fragments of epithelioid, solid neoplasia, with discrete nuclear atypia, CD34−, Chromogranin−, CD117−, HEP−, Synaptophysin−. The pathologist, however, expressed the need for typing on a more representative sample. To exclude a possible NET, the patient also performed a PET/CT scan. The examination showed a very low concentration of the marked somatostatin analog at the level of the neoformation appreciable in the retroperitoneal region and no pathological accumulations in other areas, not even in the liver. The patient performed a first surgical evaluation that did not place urgent surgical indications. The oncological evaluation expressed the need to start chemotherapy, after evaluation of the histological examination. On 26 November the patient was discharged, without further episodes of melena, with stable hemoglobin values and with an indication for oncological re-evaluation as soon as the result of the histological examination was available. The values of CEA, CA 19.9, PTH were normal.
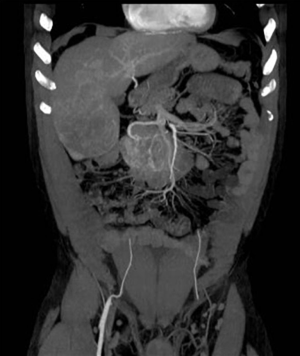
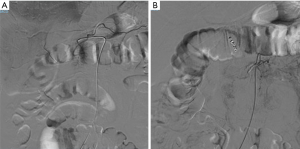
The following day, however, the patient returned to the emergency room for a new episode of melena. He was admitted to the surgery department. An angiography was performed, which showed the well-known hyper-vascularised neoformation at the mesogastric quadrant with afference from the lower pancreatic-duodenal vessels, newly formed circles, and hemorrhagic petechiae. Superselective catheterization of the Antero and posteroinferior pancreatic-duodenal afferent vessels, embolization with 250–350 micron microparticles and positioning of 5 compatible RM platinum spirals was done. The super-selective catheterization of the gastroduodenal artery was also carried out and embolized with 2 platinum spirals. At the final angiographic check, the almost complete devascularization of the mass was observed (Figure 4).
After performing the preoperative examinations and anesthesiological evaluation, on 1 December 2015 the patient underwent exploratory laparotomy surgery. In the abdominal cavity, a very extensive, rounded lesion in the liver at the VI segment and numerous other more superficial whitish lesions were found; there was also a mass of purple complexion, hyper vascularized starting from the lower margin of the III-IV duodenal portion, which extended posteriorly on the anterior margin of the inferior vena cava up to the bifurcation of the renal veins and on the left on the anterior margin of the abdominal aorta; super-posteriorly on the lower margin of the uncinate process. In addition, at the wall of the duodenum, posteriorly, a vast cavity corresponding to the erosion of the wall was digitally appreciated. The mobilization of the mass was carried out very slowly with progressive interruption of the newly formed vessels that surrounded it. After the upward separation of the posterior wall of the mass from the anterior wall of the inferior vena cava, the renal veins, as well as the anterior wall of the abdominal aorta, were exposed. Once the mass was mobilized from the retroperitoneum, it remained connected only to the duodenal wall and marginally with the lower part of the pancreas. Once this last connection was interrupted, the neoplasm was completely removed together with the duodenal wall (III-IV portion) by interrupting the duodenum upstream and downstream of the neoplasm with a mechanical stapler. The duodenal and jejunal stumps were inflected. Finally, a manual side-to-side retro-colic-jejunal duodenal anastomosis was performed in two layers. Liver segment III metastasectomy was performed for diagnostic purposes. Intra-operative cholangiography was performed to check the anatomical normality of the biliary tract (Figure 5).
The postoperative course was characterized, in 6th POD, by partial anastomotic leakage, documented with a transhepatic cholangiography and conservatively treated by trans-hepatoduodenal drainage placement. In addition, due to the appearance of anemia, he performed a super-selective angiography with evidence of hepatic artery pseudoaneurysm at the VII-VIII segment branch. This was followed by super-selective catheterization, embolization with 3 compatible platinum MR spirals and positioning of a new trans-hepatoduodenal catheter. On 24 January 2016, the patient was discharged in stable general clinical conditions, with supplementary iron therapy and indication for short-term oncological re-evaluation. The definitive histological examination confirmed a GIST. The initial oncological evaluation applied the patient to therapy with imatinib 400 mg/day. The possibility of debulking the liver mass was excluded due to the high risk of postoperative liver failure. He also performed a 6-month clinical radiological follow-up, with evidence of disease stability and good tolerance of imatinib therapy. However, about 22 months after surgery, the follow-up CT scan showed a new liver lesion. Therapy with imatinib was then increased to 800 mg/day and an indication to perform a new CEUS was given. The CEUS indicated the feasibility of a radiofrequency procedure, not before having monitored the result of the increase in therapy with imatinib. Six months after the appearance of the new liver lesion, follow up CT showed an increase in size. Therefore radiofrequency of the lesion was performed after a biopsy of the lesion. At the CEUS of follow up, the lesion remained substantially stable, with a good outcome of the radiofrequency procedure.
However, at the last follow-up, performed about 6 months after the radiofrequency, the lesion appeared bleeding, with the presence of additional adjacent metastases. In light of the general situation, an indication was given to continue therapy with imatinib 800 mg/day. At the moment the patient is waiting to perform a new CEUS for the continuation of the follow-up and for the planning of the therapeutic path.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Discussion
Our clinical case involves a young patient, with metastatic GIST and active bleeding, which caused anemia. The surgical act was necessary in order to stop bleeding, but at the same time, an R0 surgery could not be considered. Since the histological examination of the biopsy was not conclusive we could only assume that it was a GIST. The patient was, therefore, a candidate for urgent surgery, but also for possible chemotherapy, once the histological diagnosis of GIST was confirmed. Prior to the development of imatinib, there was no effective treatment for metastatic GIST and surgical resection was often attempted in the absence of any alternative or as an emergency (11).
Currently Akahoshi et al. indicate that the principal treatment strategy for a confirmed GIST is surgical resection for resectable GISTs without metastasis and administration of tyrosine kinase inhibitors (imatinib) for unresectable, metastatic, or recurrent GISTs (12). However, Liu et al. suggest that if chronic blood loss is confirmed by fecal occult blood and ECT or hemorrhage is not controlled, surgery becomes necessary and it should be performed immediately. Moreover, if patients have distant metastases at diagnosis, targeted therapy should be performed first. Similarly, therapy should also be initiated in patients with incomplete resection or high risk of recurrence after resection (3). In our case, it was not possible to start a neoadjuvant therapy since there was no histological diagnosis on the sample taken on the biopsy. Surgery therefore also became necessary for diagnosis. As reported by Gold et al. data from uncontrolled prospective trials indicate that imatinib results in a response rate of approximately 50%, with at least 75% of patients having prolonged stable disease (13) and in this way imatinib rapidly became the standard of care for the treatment of patients with metastatic GIST also in favor of its safety and tolerability (14,15). Thanks to the success of imatinib, over the years the treatment of metastatic GIST has also benefited from a second and third treatment line (sunitinib and regorafenib) and sequential treatment has more than tripled median survival (11). The patient after the surgery performed close follow-ups at 3-6 months, using CT and CEUS in line with what reported by many studies (6,12,16).
The residual liver disease was managed with non-surgical procedures and with the increase in therapy with imatinib from 400 to 800 mg as also reported by Casali et al. and Vassos et al. (6,17).
To date, follow up is still ongoing, the disease is well controlled, there is good tolerance for imatinib therapy, with a current survival of 4 years in the presence of residual non-symptomatic liver disease.
Conclusions
The GISTs can be small in size and almost asymptomatic. Sometimes, however, they may already present metastatic with major symptoms such as gastrointestinal bleeding which may require urgent treatment. Taking it for granted that in metastatic disease the standard of treatment is therapy with imatinib, in these specific urgent cases, the literature suggests short-term surgery in order to limit bleeding, and secondly start therapy with imatinib. The management of metastases, mostly hepatic or peritoneal, can also be performed with non-surgical procedures such as radiotherapy and radiofrequency, increasing the dose with imatinib or switching to a second or third line of chemotherapy (1,6,17). In these cases, the surgery is not R0 but reduces the mass and allows control of the residual disease through chemotherapy.
However, the natural history of GISTs is unknown (12) and GIST requires multidisciplinary management, which improves both prognosis and quality of life (3). For these reasons it is important to continue research and studies, both regarding management strategies in advanced cases, and the management of the residual disease.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors present the study in accordance with the CARE reporting checklist. Available at http://dx.doi.org/10.21037/acr-20-70
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/acr-20-70). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Parab TM, DeRogatis MJ, Boaz AM, et al. Gastrointestinal stromal tumors: a comprehensive review. J Gastrointest Oncol 2019;10:144-54. [Crossref] [PubMed]
- Nilsson B, Bümming P, Meis-Kindblom JM, et al. Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era--a population-based study in western Sweden. Cancer 2005;103:821-9. [Crossref] [PubMed]
- Liu Q, Kong F, Zhou J, et al. Management of hemorrhage in gastrointestinal stromal tumors: a review. Cancer Manag Res 2018;10:735-43. [Crossref] [PubMed]
- Morgan AJ, Raut CP, Duensing A, et al. Epidemiology, classification, clinical presentation, prognostic features, and diagnostic work-up of gastrointestinal stromal tumors (GIST). UpToDate 2019:1-40.
- Costa Almeida C, Caroço TV, Albano M, et al. Extragastrointestinal stromal tumour (EGIST) presented as a mesenteric and retroperitoneal mass. BMJ Case Rep 2019;12:e232481. [Crossref] [PubMed]
- Casali PG, Abecassis N, Aro HT, et al. Gastrointestinal stromal tumours: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018;29:iv267. [Crossref] [PubMed]
- Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol 2002;33:459-65. [Crossref] [PubMed]
- Vernuccio F, Taibbi A, Picone D, et al. Imaging of Gastrointestinal Stromal Tumors: From Diagnosis to Evaluation of Therapeutic Response. Anticancer Res 2016;36:2639-48. [PubMed]
- King DM. The radiology of gastrointestinal stromal tumours (GIST). Cancer Imaging 2005;5:150-6. [Crossref] [PubMed]
- Scarpa M, Bertin M, Ruffolo C, et al. A systematic review on the clinical diagnosis of gastrointestinal stromal tumors. J Surg Oncol 2008;98:384-92. [Crossref] [PubMed]
- Ford SJ, Gronchi A. Indications for surgery in advanced/metastatic GIST. Eur J Cancer 2016;63:154-67. [Crossref] [PubMed]
- Akahoshi K, Oya M, Koga T, et al. Current clinical management of gastrointestinal stromal tumor. World J Gastroenterol 2018;24:2806-17. [Crossref] [PubMed]
- Gold JS, van der Zwan SM, Gönen M, et al. Outcome of metastatic GIST in the era before tyrosine kinase inhibitors. Ann Surg Oncol 2007;14:134-42. [Crossref] [PubMed]
- Ben Ami E, Demetri GD. A safety evaluation of imatinib mesylate in the treatment of gastrointestinal stromal tumor. Expert Opin Drug Saf 2016;15:571-8. [Crossref] [PubMed]
- Reichardt P, Schlemmer M, Delgado Perez JR, et al. Safety of Imatinib Mesylate in a Multicenter Expanded Access Program in Adult Patients with Gastrointestinal Stromal Tumors in the Adjuvant Setting. Oncol Res Treat 2019;42:629-35. [Crossref] [PubMed]
- Nishida T, Hirota S, Yanagisawa A, et al. Clinical practice guidelines for gastrointestinal stromal tumor (GIST) in Japan: English version. Int J Clin Oncol 2008;13:416-30. [Crossref] [PubMed]
- Vassos N, Agaimy A, Hohenberger W, et al. Management of liver metastases of gastrointestinal stromal tumors (GIST). Ann Hepatol 2015;14:531-9. [Crossref] [PubMed]
Cite this article as: Ferro S, Fabbri N, Galeotti R, Salviato E, Cavallesco G, Pansini G. Voluminous abdominal gastrointestinal stromal tumor of unknown origin manifested with bleeding in a young man: synchronous management of the emergency and oncological approach—case report. AME Case Rep 2020;4:33.