Interstitial lung disease in dermatomyositis complicated by right ventricular thrombus secondary to macrophage activation syndrome- a case report
Introduction
Dermatomyositis (DM) is a rare connective tissue disorder that results from autoimmune antibodies by humoral immune system activation. The reported incidence of amyopathic DM (46.4%; 21%) is less as compared to classic DM and has a female preponderance (1,2). Prior to the introduction of corticosteroids in the treatment of DM, the prognosis was extremely poor, with a mortality rate as high as 50 to 61% (3,4). Muscle or skin biopsy is required to support the diagnosis; however, it is not necessary if there is a typical clinical presentation suggestive of DM. Cytokine storm known as Macrophage activation syndrome (MAS) is a fatal and uncommon complication of DM.
This case highlights MAS, which depicts a miscellaneous clinical scenario with multiple differential diagnoses which are the real challenge and can be extremely tricky to manage if the pathogenesis is not fully understood.
We report a fatal case of DM with rapidly progressive interstitial lung disease (ILD) which failed to respond to systemic steroids and consequently resulted in MAS leading to multi-organ failure and thromboembolism (right ventricular thrombus) (RVT). To the best of our knowledge, a case of DM with RVT has never been reported so far. We present the following case in accordance with the CARE guideline.
Case presentation
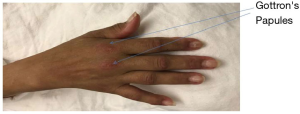
A fifty-three years old Hispanic female with a history of polyarthritis for past 3 months (with no definite diagnosis) presented in the emergency department (ED) with intermittent fever, dyspnea sore throat and dry cough for ten days. She denied chest pain, hemoptysis, rhinorrhea, nasal stuffiness, or postnasal discharge. There were no sick contacts and no history of recent travel. The patient had been experiencing arthralgias involving shoulder, elbow, wrist, interphalangeal, knee and ankle joints, burning sharp pain aggravated on movement with no relieving factor. There was erythematous rash located in periorbital areas bilateral, lateral aspect of right leg and thigh, around the neck, bilateral shoulders, left elbow, and bilateral knuckles (Figure 1). The patient had an unintentional weight loss of ten pounds for the last four months with reduced appetite.
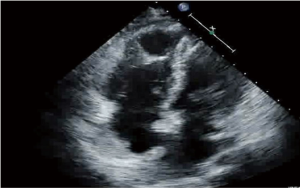
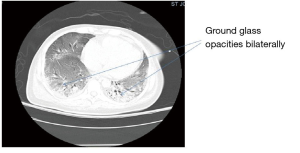
The patient was a lifetime non-smoker, non-alcoholic, never used illicit drugs, and no history of sexually transmitted diseases. No history of similar complaints and no similar history in any family member. Initial vitals in ED were, blood pressure (BP) 108/59 mmHg, heart rate 88 beats per minute, temperature 104-degree Fahrenheit, respiratory rate 30 per minute, oxygen saturation 98% with the nasal cannula at 4 liters per minute. Physical Exam was positive for mild respiratory distress, heliotrope rash, Gottron’s patches, and Shawl sign, coarse crackles on lung auscultation, equal air entry bilateral. Joints were not deformed. No organomegaly on the abdominal exam. No genitourinary rash or discharge noted. Blood work showed normocytic anemia with anisocytosis, hypocalcemia, hypophosphatemia, hypoalbuminemia, and raised aspartate aminotransferase. A chest radiogram showed increased interstitial markings on the left lower lung field. The initial diagnosis was community-acquired interstitial pneumonia. Antibiotics for atypical coverage were started. The patient deteriorated despite changing antibiotics; in the meantime, blood cultures and sputum culture were negative. Throat swab was negative for Streptococcus pneumonia. Arterial blood gas showed fully compensated respiratory alkalosis while on 100% oxygen on a non-rebreather mask. A computed tomography (CT) scan of the chest showed the ground-glass appearance of the interstitium (Figure 2). CT angiography ruled out pulmonary embolism. A high dose of systemic steroid was started (80 mg/day). Subsequently, the patient became hypoxemic and hypotensive and had to be placed on mechanical ventilation. Ferritin 1,600 ng/mL, lactate dehydrogenase (LDH) was 490 Units/Liter, erythrocyte sedimentation rate (ESR) was 60 mm/hour, C-reactive protein (CRP) was 176 mg/L. Thereafter acute renal failure set in along with bicytopenia (anemia and thrombocytopenia). Continuous veno-venous hemofiltration was initiated. A transesophageal echocardiogram revealed a thrombus in the right ventricle (Figure 3). There was no evidence of deep vein thrombosis on the Doppler ultrasound of extremities.
There was evidence of myositis [high creatine kinase 279 U/L and myoglobin: 142 ng/mL, although aldolase (6.7 U/L) was normal; TSH (0.54 mIU/L) was within normal limits. Anti clinically amyopathic dermatomyositis (CADM) 140 antibody was positive; the rest of the autoimmune panel was unremarkable; antinuclear antibodies, anti-smith antibodies, anti-double-stranded deoxyribonucleic acid antibodies, anti-jo-1 antibody, anti-smooth muscle, rheumatoid factor, antineutrophil cytoplasmic antibodies were negative. Complement levels (C3 and C4) were within normal limits, 128mg/dL and 34mg/dL respectively. Human immunodeficiency virus serology was negative. Lyme serology was negative. The hepatitis C antibody was negative. The lupus anticoagulant was negative. Prothrombin time (PT) and activated partial thromboplastin time (aPTT) was mildly elevated (PT 16.1 s, aPTT 38.7 s), and international normalized ratio (INR) was normal 1.3. Fibrinogen was normal (274 mgm %), fibrin degradation products were elevated (>20 µgm/mL), D-dimer was >20 µgm/ml. Peripheral blood film did not show schistocytes. LDH was elevated; however, the haptoglobin level was normal. The direct Coombs test was negative. Heparin-induced thrombocytopenia panel was negative. CT brain showed diffuse cerebral edema. Hypotension was refractory to vasopressors. The patient expired on day tenth of hospitalization (for details of the course see Figure 4).

Discussion
DM population is prone to development ILD, more so in the presence of factors like older age at diagnosis, arthritis or arthralgias, fever, presence of anti-Jo-1 antibodies, elevated ESR, presence of anti-MDA5 antibodies, and elevated CRP level. In a genetic study on the Chinese Han population involving 1017 patients with DM/PM, it was observed that there is a positive association between ANKRD5 gene polymorphism and DM-ILD (5,6).
Thrombosis in autoimmune disease is multifactorial. The overall incidence of pulmonary embolism in DM is three times more as compared to the general population; risk increases to sixteen folds for a one year follow up (7-10). Humoral immune response flare mediates Microangiopathic vascular damage that sets in a cascade of thrombogenesis. Risk is augmented with comorbid conditions and other systemic diseases (11,12). There is evidence suggesting a strong association between thromboembolism that includes deep vein thrombosis and pulmonary embolism, in DM (13). A most common presentation is deep vein thrombosis or pulmonary embolism; however, our patient developed a right ventricular thrombus (7,14-17).
Thrombosis can be best explained by systemic inflammation by an autoimmune attack. There is induction of tissue factor expression and inhibition of fibrinolysis by upregulation of plasminogen activator inhibitor levels (18). There is multi-system involvement like a cardiovascular, pulmonary, hematological, and central nervous system. Severity and progression of ILD depend on patient factors like age, skin rash, presence of anti Jo antibodies.
The prothrombotic state is also attributed to inflammatory mediators like interleukin six which are responsible for reducing levels of protein S, and TNF alpha which downregulates endothelial protein C receptor and thrombomodulin to experimental studies suggest that (19). Once ILD starts to develop, the disease can progress rapidly and is highly fatal. There are various types of ILD based on high resolution computed tomography features (HRCT) like non-specific interstitial pneumonia, lymphocytic interstitial pneumonia, cryptogenic, and acute interstitial pneumonia. Our patient had a ground-glass appearance along with bilateral lower lobar interstitial pneumonia, consistent with non-specific interstitial pneumonia.
Multi-system failure has been attributed to MAS, which is phagocytosis of hematopoietic elements by activated macrophages. Mostly seen in association with rheumatoid arthritis and lupus; however, it has been reported in several cases of juvenile DM. MAS leads to a multi-system failure that includes cytopenias, thrombosis, renal failure, heart failure. Major Cytokines involved are Interleukin 6, 8, 18, tumor necrosis factor-alpha, and interferon-gamma. MAS can result from triggers like infections (viral/bacterial/parasitic), malignancy (like lymphocytic leukemia and Hodgkin’s lymphoma), autoimmunity and environmental factors, and lung transplantation. In such cases, it is better known as a reactive or secondary hemophagocytic syndrome (HPS). HPS leads to a significant spill of cytokines from activated macrophages which attacks and destroys host tissue.
In our case, the most likely underlying cause of right ventricular thrombus was primary MAS. Although the patient had positive Streptococcal pneumoniae IGG antibodies (IgM antibodies were negative) and hepatitis B core antibodies positive, both reflect past infection.
The patient was on systemic steroids since the diagnosis (one month) of DM. There was minimal relief in arthralgias. Thereafter patient presented with non-specific symptoms of suggestive of pneumonia. It is crucial to have a complete understanding of inflammatory myopathies as their presentation can mimic sepsis. Fever, cough, dyspnea, and lung involvement in radiogram can easily be confused with bacterial or viral pneumonia.
The diagnosis is based on a collaboration between clinical and laboratory parameters. There are no established diagnostic criteria for MAS/HPS, however, researchers have designed criteria that have been validated. There are several criteria reported for MAS is in association with juvenile idiopathic arthritis (JIA), lupus, and adult onset Still’s disease and systemic sclerosis. PRINTO criteria are one such criteria (designed for JIA) Sensitivity of 72 percent and specificity of 97 percent; it was designed after a multi-process approach involving a questionnaire answered by expert consultants in rheumatology (20).
There is no criterion specific for MAS diagnosis in DM; however, the basic mechanism of MAS is similar in all connective tissue and autoimmune disorders; therefore, the diagnosis can be established keeping in consideration multi-system involvement. Laboratory findings include cytopenias, coagulopathy, hypofibrinogenemia, hyperferritinemia, hypertriglyceridemia, hypoalbuminemia, and abnormal liver enzymes. High ferritin relates to resistance to response to therapy and worse outcome compared to low or normal ferritin levels. In the presence of high ferritin, low glycosylated ferritin is a known prognostic factor. Bone marrow may show active phagocytosis of hematopoietic elements by phagocytes; however, negative bone biopsy does not preclude the diagnosis of MAS. Biopsy from other organs like lymph nodes, liver, and spleen may show similar histopathological features. Testing for specific genetic expression is a useful diagnostic tool; however, not utilized commonly as it is not readily available in all centers.
In our case, MAS rapidly spread to involve almost every organ system and was resistant to therapy. Such patients must be heparinized and treated with high dose corticosteroids, tacrolimus, cyclosporine, etoposide, intravenous immunoglobulin. Plasmapheresis is the last resort for unresponsive cases (21-25). Given the diagnostic difficulty of MAS and the patient’s disease evolution, recognizing MAS is often delayed. Furthermore, having a bone marrow biopsy early in the course of the disease could have guided further treatment, including expanding to cytokine specific therapies. Establishing validated diagnostic criteria for MAS, as well as further clinical trials, are needed to aid in early treatment options. Familial cases are Primary MAS and have a better prognosis compared to secondary. Increased risk of systemic thromboembolism is secondary to systemic inflammation which up-regulates procoagulants in blood with simultaneous suppressing fibrinolysis. Inflammatory myopathies have been reported to cause deep vein thrombosis, pulmonary embolism, and cerebral thromboembolism; however, MAS with right ventricular thrombus is a unique finding in our case.
Conclusions
Rapidly progressive ILD in a patient with autoimmune myopathy like DM is a dreaded complication of the disease. In the case of rapidly deteriorating ILD, one must anticipate MAS and evaluate for thromboembolism along with aggressive initiation therapy.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/acr.2020.03.06). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ang P, Sugeng MW, Chua SH. Classical and amyopathic dermatomyositis seen at the National Skin Centre of Singapore: a 3-year retrospective review of their clinical characteristics and association with malignancy. Ann Acad Med Singapore 2000;29:219-23. [PubMed]
- Bendewald MJ, Wetter DA, Li X, et al. Incidence of dermatomyositis and clinically amyopathic dermatomyositis: a population-based study in Olmsted County, Minnesota. Arch Dermatol 2010;146:26-30. [Crossref] [PubMed]
- Marie I. Morbidity and mortality in adult polymyositis and dermatomyositis. Curr Rheumatol Rep 2012;14:275-85. [Crossref] [PubMed]
- Airio A, Kautiainen H, Hakala M. Prognosis and mortality of polymyositis and dermatomyositis patients. Clin Rheumatol 2006;25:234-9. [Crossref] [PubMed]
- Li L, Chen S, Wen X, et al. Positive Association between ANKRD55 Polymorphism 7731626 and Dermatomyositis/Polymyositis with Interstitial Lung Disease in Chinese Han Population. Biomed Res Int 2017;2017:2905987.
- Gri G, Piconese S, Frossi B, et al. CD4+CD25+ regulatory T cells suppress mast cell degranulation and allergic responses through OX40-OX40L interaction. Immunity 2008;29:771-81. [Crossref] [PubMed]
- Choi HK, Rho YH, Zhu Y, et al. The risk of pulmonary embolism and deep vein thrombosis in rheumatoid arthritis: a UK population-based outpatient cohort study. Ann Rheum Dis 2013;72:1182-7. [Crossref] [PubMed]
- Xu J, Lupu F, Esmon CT. Inflammation, innate immunity and blood coagulation. Hamostaseologie 2010;30:5-6, 8-9. [Crossref] [PubMed]
- Zöller B, Li X, Sundquist J, et al. Risk of pulmonary embolism in patients with autoimmune disorders: a nationwide follow-up study from Sweden. Lancet 2012;379:244-9. [Crossref] [PubMed]
- Ramagopalan SV, Wotton CJ, Handel AE, et al. Risk of venous thromboembolism in people admitted to hospital with selected immune-mediated diseases: record-linkage study. BMC Med 2011;9:1. [Crossref] [PubMed]
- Carruthers EC, Choi HK, Sayre EC, et al. Risk of deep venous thrombosis and pulmonary embolism in individuals with polymyositis and dermatomyositis: a general population-based study. Ann Rheum Dis 2016;75:110-6. [Crossref] [PubMed]
- Smeeth L, Cook C, Thomas S, et al. Risk of deep vein thrombosis and pulmonary embolism after acute infection in a community setting. Lancet 2006;367:1075-9. [Crossref] [PubMed]
- Lee YH, Song GG. Idiopathic inflammatory myopathy and the risk of venous thromboembolism: a meta-analysis. Rheumatol Int 2017;37:1165-73. [Crossref] [PubMed]
- Selva-O'Callaghan A, Fernández-Luque A, Martínez-Gómez X, et al. Venous thromboembolism in patients with dermatomyositis and polymyositis. Clin Exp Rheumatol 2011;29:846-9. [PubMed]
- Johannesdottir SA, Schmidt M, Horváth-Puhó E, et al. Autoimmune skin and connective tissue diseases and risk of venous thromboembolism: a population-based case-control study. J Thromb Haemost 2012;10:815-21. [Crossref] [PubMed]
- Naess IA, Christiansen SC, Romundstad P, et al. Incidence and mortality of venous thrombosis: a population-based study. J Thromb Haemost 2007;5:692-9. [Crossref] [PubMed]
- Aviña-Zubieta JA, Abrahamowicz M, Choi HK, et al. Risk of cerebrovascular disease associated with the use of glucocorticoids in patients with incident rheumatoid arthritis: a population-based study. Ann Rheum Dis 2011;70:990-5. [Crossref] [PubMed]
- Zöller B, Li X, Sundquist J, Sundquist K. Autoimmune diseases and venous thromboembolism: a review of the literature. Am J Cardiovasc Dis 2012;2:171-83. [PubMed]
- Rahman P, Inman RD, El-Gabalawy H, et al. Pathophysiology and pathogenesis of immune-mediated inflammatory diseases: commonalities and differences. J Rheumatol Suppl 2010;85:11-26. [Crossref] [PubMed]
- Lazarevic D, Pistorio A, Palmisani E, et al. The PRINTO criteria for clinically inactive disease in juvenile dermatomyositis. Ann Rheum Dis 2013;72:686-93. [Crossref] [PubMed]
- Hu S, Bansal P, Lynch D, et al. Rituximab, etoposide, methylprednisolone, high-dose cytarabine, and cisplatin in the treatment of secondary hemophagocytic lymphohistiocytosis with classical Hodgkin lymphoma: a case report and review of the literature. J Med Case Rep 2016;10:365. [Crossref] [PubMed]
- Teshigawara S, Katada Y, Maeda Y, et al. Hemophagocytic lymphohistiocytosis with leukoencephalopathy in a patient with dermatomyositis accompanied with peripheral T-cell lymphoma: a case report. J Med Case Rep 2016;10:212. [Crossref] [PubMed]
- Qushmaq KA, Chalmers A, Esdaile JM. Cyclosporin A in the treatment of refractory adult polymyositis/dermatomyositis: population based experience in 6 patients and literature review. J Rheumatol 2000;27:2855-9. [PubMed]
- Kaieda S, Yoshida N, Yamashita F, et al. Successful treatment of macrophage activation syndrome in a patient with dermatomyositis by combination with immunosuppressive therapy and plasmapheresis. Mod Rheumatol 2015;25:962-6. [Crossref] [PubMed]
- Kwa MC, Ardalan K, Laumann AE, et al. Predictors of Hospitalization, Length of Stay, and Cost of Care Among Adults With Dermatomyositis in the United States. Arthritis Care Res (Hoboken) 2017;69:1391-9. [Crossref] [PubMed]
Cite this article as: Paul N, Avalos C, Estifan E, Swyden S. Interstitial lung disease in dermatomyositis complicated by right ventricular thrombus secondary to macrophage activation syndrome- a case report. AME Case Rep 2020;4:18.