Case report: left monoplegia in acute type B aortic dissection
Introduction
An acute type B aortic dissection (ATBAD) with malperfusion is a devastating complication. Ischemic symptoms in ATBAD occur in the arm or legs in 20% of patients, in the kidney in 15%, in the myocardium in 10%, in the brain in 5%, and in the mesentery or spinal cord in 3% (1). However, despite the possibility of various arterial involvement in ATBAD, cases of monoplegia due to spinal cord ischemia is extremely rare. Additionally this complication may progress to irreversible and complete paraplegia without urgent appropriate interventions. Furthermore, effective treatments for malperfusion induced spinal cord ischemia have not been established yet (2). So, we would like to introduce a case of a 62-year-old man who presented with left monoplegia induced malperfusion of spinal cord in ATBAD and treated with endovascular fenestration.
We present this following case in accordance with the CARE guideline (3).
Case presentation
A 62-year-old man with hypertension presented with a sudden onset of chest pain with numbness and weakness of the left lower extremity. Neurologic examination revealed left lower extremity monoplegia (motor grade 5/0). Follow up CT scan demonstrated ATBAD beginning below the left subclavian artery and extending to the level of iliac bifurcation without distal reentry, involving malperfusion of the left renal artery, left intercostal and left lumbar branches. Considering the urgency and comorbidity, surgical repair and endovascular stent graft were ruled out, finally endovascular fenestration was performed.
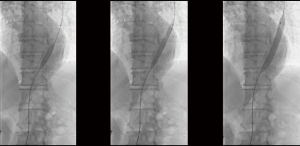
Under local analgesia, the right common femoral artery was catheterized and a 5Fr sheath catheter was positioned into the true lumen (Cook Medical, IN, USA). An aortogram confirmed that the catheter was within the compressed true lumen of the aortic dissection. At the level of celiac trunk, aortic fenestration ballooning was performed to enlarge a tearing site by using first 12-mm and then 20-mm diameter balloons (Boston Scientific, Natick, Mass) (Figure 1).
Cerebrospinal drainage couldn’t be performed prior to endovascular fenestration because heparin was already injected, which may have increased cerebrospinal hemorrhagic risk and his motor and sensory function are recovered within 30 minutes of the endovascular fenestration as well.
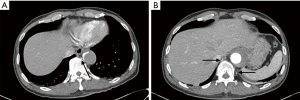
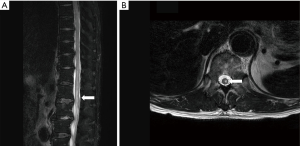
After endovascular fenestration, final angiography was demonstrated increased flow in the true lumen of the descending aorta with good patency of the left renal artery where no flow had been observed (Figure 2). Enhanced CT confirmed the recovery of flow to the left intercostal and lumbar branches. The patient achieved the complete recovery of sensory function of his left leg. His neurologic examination results were also normalized, showing hip and right knee flexion, ankle extension. On postoperative day (POD) 3, he walked using a q-cane. Magnetic resonance imaging on POD7 reconfirmed the preoperative spinal cord ischemia, showing that left asymmetric increased T2 signal intensity of the spinal cord from T11 to L2 level (Figure 3). He is being followed up on an outpatient basis and shows no complications.
Discussion
ATBAD with malperfusion is a devastating complication. Estrera et al. reported that paraplegia occurred in 8.5% of ATBAD patients and most patients involving bilateral low extremities in 81% (4). However, despite the possibility of arterial involvement in ATBAD, cases of monoplegia due to spinal cord ischemia are extremely rare. This spinal cord ischemia may progress to irreversible and complete paraplegia in the absence of appropriate urgent interventions. To avoid its catastrophic prognosis, appropriate treatments for reperfusion flow to the ischemic spinal cord are required. However, because of low incidence rate of distal extremity ischemia, most vascular surgeons can’t easily impress this spinal cord ischemia in ATBAD. Before confirming spinal cord ischemia, they estimate the dissecting malperfusion of peripheral blood flow and initially provide routine thrombolytic procedures which might make the patients’ treatment more difficulty as being more bleeding tendency.
Malperfusion in ATBAD can be diagnosed based on CT findings consistent with reduced or absent flow to an end-organ or complete true lumen collapse, including disappearance of the aortic double lumen, indicating elimination of the true lumen with the dissection flap being pushed against the aortic wall causing obstruction of flow to branch vessels, continuation of dual lumen patency with absent flow in a branch vessel (dynamic malperfusion) and dissection into a branch vessel or a thrombosed false lumen in a branch vessel (static malperfusion), with or without clinical evidence of end-organ dysfunction (5).
Endovascular fenestration between the true and false lumens can be performed with various techniques, with the goal of equalizing the systolic pressures between the 2 chambers and decompressing the pressurized false lumen (6). Additionally, the advantages of endovascular fenestration are directly relieving organ or limb ischemia in a faster way than by aortic graft replacement (7). Among the various endovascular fenestration techniques, the funnel technique enlarges the surface area of the re-entry orifice in the true lumen with an endoaortic stent positioned between the 2 lumens, which increases flow and pressure in the true lumen and reduces them in the false lumen, thus improving the efficacy of fenestration. This technique can be an effective treatment (clinical success: 89%) associated with low morbidity (30-day mortality: 7%, major complications rate: 3.6%) (8).
Conclusions
We contemplated which one among endovascular fenestration and TEVAR is more appropriate approach. TEVAR for central repair was determined to not be suitable for landing because the left subclavian artery is too close at the tearing site and the arch diameter is too small (38 mm). Finally, endovascular fenestration was chosen, and the result was satisfactory. Therefore, we would like to introduce this rare case of left lower extremity monoplegia with ATBAD and suggest that endovascular fenestration can be an effective treatment option to treat spinal cord ischemia in ATBAD.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/acr.2020.03.05). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this Case report and any accompanying images. The submission version of the report was read by the patient, and the reports’ content was confirmed as being correct to the best of his knowledge.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mesazro I, Morocz J, Szlavi J, et al. Epidemiology and clinicopatholgy of aortic dissection. A population-based longitudinal study over 27 years. Chest 2000;117:1271-8. [Crossref] [PubMed]
- Fujisawa Y, Morishita K, Fukada J, et al. Treatment Method for spinal cord injury caused by Acute Type B Aortic Dissection. Asian Cardiovasc & Thoracic Ann 2006;14:e106-7. [Crossref]
- Rily Ds, Barber MS, Kienle GS, et al. CARE 2013 Explanations and Elaborations: Reporting Guidelines for Case Reports. J Clin Epidemiol 2017;89:218-35. [PubMed]
- Estrera AL, Miller CC 3rd, Safi HJ, et al. Outcomes of Medical Management of Acute Type B Aortic dissection. Circulation 2006;114:I384-9. [Crossref] [PubMed]
- Norton EL, Williams DM, Kim KM, et al. Management of acute type B dissection with malperfusion via endovascular fenestration/stenting. J Thorac Cardiovasc Surg 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Preventza O. Commentary: Fenestration in static malperfusion for acute type B aortic dissection: Teamwork can be the Holy Grail, but concerns remain. J Thorac Cardiovasc Surg 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Veger HTC, Pasveer EH, Visser MJT. Where to fenestrate in aortic dissection type B? An Ex-vivo study. Ann Vasc Surg 2017;43:296-301. [Crossref] [PubMed]
- Vendrell A, Frandon J, Rodiere M, et al. Aortic dissection with acute malperfusion syndrome: Endovascular fenestration via the funnel technique. J Thorac Cardiovasc Surg 2015;150:108-15. [Crossref] [PubMed]
Cite this article as: Kim H, Heo W, Song SW, Yoo KJ. Case report: left monoplegia in acute type B aortic dissection. AME Case Rep 2020;4:16.