
Viral pneumonia and pulmonary alveolar proteinosis: the cause and the effect, case report
Introduction
Pulmonary alveolar proteinosis (PAP) is a rare lung disease characterized by abnormal accumulation of lipoproteinaceous surfactant component in the alveolar space. It has an incidence and prevalence of 0.36 and 3.7 cases per million respectively (1). There are four clinically distinct forms of PAP, the autoimmune (idiopathic) type being the commonest (90% of cases). This type of PAP is characterized by deficiency in granulocyte macrophage-colony stimulating factor (GM-CSF), as a result of the anti-GM-CSF antibody production, which results into impaired surfactant clearance that leads to the accumulation of surfactant in the alveolar space (2). The interaction between different pathogens and PAP is a complex issue. The dysfunction of alveolar macrophages and neutrophils is documented to increase the risk of opportunistic infections; furthermore, it has been shown that various infections can influence the disease onset and alter its course (1,3). In this report we present a newly diagnosed case of autoimmune PAP (APAP), which was thought to be elicited by a viral pneumonia, and furthermore, had experienced an acute clinical deterioration “exacerbation” that found to be triggered by a confirmed H1N1 influenza infection.
Case presentation
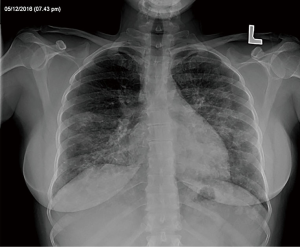
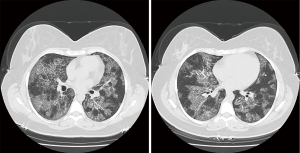
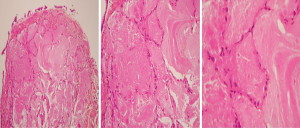
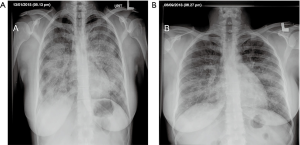
A 38-year-old ex-smoker woman who was first seen for progressive dyspnea and cough that had lasted 4 months. She received treatment for CAP and had improved partially. At this stage of the illness no bacteriological nor viral pathogens work up was requested. Later, she was referred to our hospital due to her sub-optimal clinical response. She left with exertional dyspnea, subjective fever and chills as well as persistent patchy interstitial and airspace opacities on chest X-ray (CXR). Her past medical history was unremarkable, and she never had flu vaccine before. On physical examination, she was stable and not in respiratory distress. Her oxygen saturation was 94% on room air and her lungs auscultation was remarkably clear. Chest radiographic image is shown in (Figure 1). Pulmonary function tests revealed restrictive defect and disproportionate reduction in diffusing capacity (DLCO). Laboratory findings included a normal complete blood count though her Hb was 16 g/dL, an elevated lactate dehydrogenase (LDH) level of 300 U/L. Microbiologic and serologic tests for fungal infections, tuberculosis, HIV and autoimmune diseases were all negative. A computed tomographic scan of the chest is shown in (Figure 2). The patient underwent bronchoscopy, her bronchoalveolar lavage (BAL) fluid was milky opaque in color. Results for the cytologic specimens with special stains for microorganisms were all negative. However, BAL analysis showed morphologically abnormal “foamy” macrophages engorged with intracellular inclusions on a background of granular acellular eosinophilic proteinaceous material that stains positive with periodic acid Schiff (PAS). The transbronchial lung biopsies (TBB) result is shown in (Figure 3). A diagnosis of PAP was made and she underwent recurrent sequential whole lung lavage (WLL) over 9 months (Figure 4). During subsequent follow up visits, the serum anti-GM-CSF antibody test result came strongly positive, supporting our diagnosis. She responded to the therapy and her symptoms improved with a residual mild shortness of breath when rushing at work. During the winter season of 2018 she experienced acute deterioration of her condition, that was associated with flu-like symptoms. Her oxygen saturation was 88% on room air and dropped further to 75% while walking. Her chest was full of crackles and her CXR showed worsened opacities with more diffuse infiltrates (Figure 5A). Nasopharyngeal swabs with viral reverse transcription polymerase chain reaction (RT-PCR) were positive for H1N1. The rest of other bacterial and viral panel came to be negative. She was admitted to intensive care unit and treated with broad spectrum antibiotics and oseltamivir. Her condition had improved significantly, however, was unsuitable for repeated WLL and so she was discharged home on oxygen treatment. Recombinant GM-CSF was offered as a therapeutic option, however, she declined it with no clear reason. With follow-up, her shortness of breath and cough have improved, with less sputum. Her oxygen saturation is maintained at rested, however, she uses oxygen while walking. Although her PFTs were not changed but her chest radiograph has significantly improved (Figure 5B).
Discussion
PAP is a rare disease that is three times more common in males, and approximately two thirds of patients with this condition are current smokers (1). Clinically, patients usually present with insidious progressive dyspnea and cough that may be associated with fever, chest pain, or hemoptysis. Auscultation of the chest may reveal fine crackles but is more frequently unrevealing. With these non-specific symptoms, it is not surprising that many patients are misdiagnosed with CAP. In fact, it has been shown that the median delay between symptoms onset and diagnosis was seven months (1).
The relation between infectious pneumonia and PAP is complex and worth further studies in order to be better understood. The accumulation of intra-alveolar PAS-positive material in patients with underlying pulmonary condition such as infection is referred to as secondary PAP (4). Pathogens that reported to cause secondary PAP include Pneumocystis jirovecii, Nocardia, Mycobacterium tuberculosis, cytomegalovirus (CMV), parainfluenza virus 3 and Epstein-Barr virus (EBV) (5-8). Other conditions causing PAP include hematologic cancers, inhalation of inorganic dust or toxic fumes and immunodeficiencies. In the other hand, the primary APAP constitute more than 90% of cases, where antibodies to GM-CSF neutralize the effect of GM-CSF on alveolar macrophages. Small percentage of cases are either congenital PAP, “an autosomal recessive disease” or unclassified PAP, “when there is no evidence of anti-GM-CSF antibodies and no clear secondary causes” (9).
Studies have shown that alveolar macrophages from knockout mice without the GM-CSF gene have a number of functional defects, including defects in cellular adhesion, expression of pathogen-associated molecular pattern receptors, toll-like receptor signaling, phagocytosis of pathogens, intracellular killing of bacteria (independent of uptake), pathogen-stimulated secretion of cytokines, and Fc receptor-mediated phagocytosis (2). Moreover, the alveolar filling lipoproteinaceous material is an excellent medium for microbial growth and hence it frequently becomes superimposed by respiratory pathogens (4,7). As a result, patients with APAP are at increased risk of various infections. Interestingly, although the presence of circulating anti-GM-CSF antibodies is thought to be idiopathic, however, the role of probable viral infection triggering these antibodies was not confirmed nor ruled out. Reported cases include infection with Streptococcus and Staphylococcus as well as Gram negative bacteria such as Klebsiella, Haemophilus, Pseudomonas, Serratia, Proteus, and Escherichia coli (1). In addition, these patients are more prone for opportunistic infection such as Nocardia, Mycobacterium tuberculosis, Mycobacterium avium-intracellulare, Pneumocystis jirovecii, Aspergillus species and various other fungi (1). Infections have been reported to be associated with PAP in 5–20% of the cases (10). Singh and his colleagues attempts to distinguish primary from secondary PAP; they found that the intra-alveolar material in patients with primary alveolar proteinosis stained uniformly for surfactant specific apoprotein, whereas the staining was focal in patients with secondary PAP (11). Nevertheless, identification of the primary disease process in cases of coexistent APAP and pulmonary infection is often difficult, which makes us to ask which one is the cause and which one is the effect? Detection of an autoimmune antibody is not synonymous with idiopathic etiology; for example, inhalational exposures to silica can trigger autoimmune scleroderma (12,13). Studies have shown that 26% to 34.2% of patients with APAP had a history of occupational inhalational exposure (14,15). Our case is definitely diagnosed to have APAP as it is a biopsy-proven case with positive serum anti-GM-CSF antibody. The coexistence of infection in the initial presentation of the case could not be identified nor excluded; she never had flu vaccine and tests to diagnosis the causative pathogen of the presumed CAP was not pursued. The superimposed infections usually cause significant clinical deterioration as observed in our case. It has been shown that 20% of the mortality due to PAP is secondary to infection (1). Contrary, remissions of APAP have been reported in cases that developed local (pneumonia) or systemic (encephalitis) infection (3). Remission of APAP reported to occur following viral or bacterial infection (3). It has been hypothesized that remission of APAP is triggered by the induction of GM-CSF following the infection (3). The APAP clinical course can improve after treatment of the underlying bacterial or fungal infection (3,16,17). This can be due to abolishing suppressive effect of certain types of pathogens (as Aspergillus) on the macrophage function (3,18).
Worldwide, seasonal influenza estimated to affect about 3 to 5 million and cause 290,000 to 650,000 deaths annually (19). GM-CSF paly crucial role in the immune response to influenza by enhancing innate immune mechanisms that depend on alveolar macrophages and hence reduce the morbidity and mortality due to influenza virus (20). In spite the impaired macrophage function in APAP, clinical deterioration during influenza season is not well described in the literature. H1N1 influenza pneumonia complicating APAP is not a common issue, it was described before only in a single case report from Italy (10). This may be explained by publication bias favouring the reporting of unusual opportunistic organisms or could be due to underdiagnosing of Influenza in APAP due to lack of prior clinical suspicion and testing (1,14). Nonetheless, our case describes both possible viral infection causation of a newly diagnosed APAP and a confirmed H1N1 superinfection for the same patient few months later. So that and to the best of our knowledge, this is the first report that describes this issue. In fact, in both cases the antiviral therapy with oseltamivir were prescribed and had led to marked improvement. The role of bronchopulmonary lavage during the acute infectious worsening of APAP is unclear as only a few case reports describe favorable results (21). In the view of sever hypoxemia during infectious deterioration of APAP the standard WLL poses significant morbidity risk, in Nicolini and Barlascini’s H1N1 case, they used a modified lobar lavage technique using bronchofibroscopy (10).
Conclusions
PAP is a rare disease that can be triggered by infections. The commonest type of PAP is the autoimmune type where anti-GM-CSF antibody causes macrophage dysfunction that results into impaired surfactant clearance and leading to its accumulation. The role of possible viral causation “trigger” or cross-reactivity of these antibodies need to be studied. The dysfunction of the immune response along with presence of lipoproteinaceous material facilitate pathogen superinfection. Superimposed infections can alter the natural history of APAP in one of the three ways; it can either cause APAP to flare up, remission or improve after the treatment of the infection. Few cases have described influenza infections complicating APAP despite the of the impaired function of the alveolar macrophages which have a biovital defensive role against this virus. Our case shows that Influenza infection should be considered during an acute deterioration of APAP cases. Antiviral therapy with Oseltamivir seems to be beneficial in such exacerbations.
Acknowledgment
The authors would like to thank the patient for participating and the thoracic surgery team members as they are crucial to providing good clinical care.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of this written consent is available for review by the Editor-in-Chief of this journal.
References
- Seymour JF, Presneill JJ. Pulmonary alveolar proteinosis: progress in the first 44 years. Am J Respir Crit Care Med 2002;166:215-35. [Crossref] [PubMed]
- Trapnell BC, Whitsett JA, Nakata K. Pulmonary alveolar proteinosis. N Engl J Med 2003;349:2527-39. [Crossref] [PubMed]
- Kobayashi T, Arai T, Hirose M, et al. Temporary remission of autoimmune pulmonary alveolar proteinosis after infectious episodes. Sarcoidosis Vasc Dif 2016;35:85-90.
- Prakash UB, Barham SS, Carpenter HA, et al. Pulmonary alveolar phospholipoproteinosis: experience with 34 cases and a review. Mayo Clin Proc 1987;62:499-518. [Crossref] [PubMed]
- Brach BB, Harrell JH, Moser KM. Alveolar proteinosis. Lobar lavage by fiberoptic bronchoscopic technique. Chest 1976;69:224-7. [Crossref] [PubMed]
- Huizar I, Kavuru MS. Alveolar proteinosis syndrome: pathogenesis, diagnosis, and management. Curr Opin Pulm Med 2009;15:491-8. [Crossref] [PubMed]
- Butnor KJ, Sporn TA. Human parainfluenza virus giant cell pneumonia following cord blood transplant associated with pulmonary alveolar proteinosis. Arch Pathol Lab Med 2003;127:235-8. [PubMed]
- Edwards C, Primhak R, Cohen MC. Pulmonary alveolar proteinosis associated with Epstein-Barr virus infection. Eur Respir J 2010;36:1214-6. [Crossref] [PubMed]
- Teja K, Cooper PH, Squires JE, et al. Pulmonary alveolar proteinosis in four siblings. N Engl J Med 1981;305:1390-2. [Crossref] [PubMed]
- Nicolini A, Barlascini C. Lobar flexible fiberoptic lung lavage: therapeutic benefit in severe respiratory failure in pulmonary alveolar proteinosis and influenza A H1N1 pneumonia. Clin Pract 2011;1:e53. [Crossref] [PubMed]
- Singh G, Katyal SL, Bedrossian CW, et al. Pulmonary alveolar proteinosis. Staining for surfactant apoprotein in alveolar proteinosis and in conditions simulating it. Chest 1983;83:82-6. [Crossref] [PubMed]
- Rubio-Rivas M, Moreno R, Corbella X. Occupational and environmental scleroderma. Systematic review and meta-analysis. Clin Rheumatol 2017;36:569-82. [Crossref] [PubMed]
- Ronsmans S, Nemery B. The presence of autoimmune antibodies in pulmonary alveolar proteinosis does not necessarily imply idiopathic disease. Lancet Respir Med 2018;6:e48. [Crossref] [PubMed]
- Inoue Y, Trapnell BC, Tazawa R, et al. Characteristics of a large cohort of patients with autoimmune pulmonary alveolar proteinosis in Japan. Am J Respir Crit Care Med 2008;177:752-62. [Crossref] [PubMed]
- Xiao YL, Xu KF, Li Y, et al. Occupational inhalational exposure and serum GM-CSF autoantibody in pulmonary alveolar proteinosis. Occup Environ Med 2015;72:504-12. [Crossref] [PubMed]
- Lin KP, Sheng WH, Wang CP, et al. Resolution of secondary pulmonary alveolar proteinosis following treatment of rhinocerebral aspergillosis. Int J Infect Dis 2010;14 Suppl 3:e246-9. [Crossref] [PubMed]
- Yamaguchi S, Takayanagi N, Tokunaga D, et al. A case of pulmonary alveolar proteinosis which initially deteriorated rapidly with exacerbation of pulmonary nocardiosis, responded promptly to treatment of the pulmonary nocardiosis. Nihon Kokyuki Gakkai Zasshi 2010;48:580-3. [PubMed]
- Murayama T, Amitani R, Ikegami Y, et al. Suppressive effects of Aspergillus fumigatus culture filtrates on human alveolar macrophages and polymorphonuclear leucocytes. Eur Respir J 1996;9:293-300. [Crossref] [PubMed]
- World health organization. Accessed on 17/04/2019. Available online: http://www.who.int/mediacentre/factsheets/fs211/en/
- Huang FF, Barnes PF, Feng Y, et al. GM-CSF in the lung protects against lethal influenza infection. Am J Respir Crit Care Med 2011;184:259-68. [Crossref] [PubMed]
- Pascual J, Gómez Aguinaga MA, Vidal R, et al. Alveolar proteinosis and nocardiosis: a patient treated by bronchopulmonary lavage. Postgrad Med J 1989;65:674-7. [Crossref] [PubMed]
Cite this article as: Albogami SM, Touman AA. Viral pneumonia and pulmonary alveolar proteinosis: the cause and the effect, case report. AME Case Rep 2019;3:41.



