
The primary pulmonary NUT carcinomas and some uncommon somatic mutations identified by next-generation sequencing: a case report
Introduction
NUT midline carcinoma (NMC) is a rare and highly aggressive carcinoma, sharing some histopathologic characteristics with squamous cell carcinomas (1). From the first case reported by Kubonishi et al. in 1991 (2), it has attached much interest. In contrast to other squamous cell carcinomas, NMC is defined by a chromosomal rearrangement involving the gene nuclear protein in testis (NUT; also known as NUTM1) on chromosome 15, contributing to the fusion of NUT to bromodomain-containing protein 4 (BRD4) in most cases and bromodomain-containing protein 3 (BRD3) in minor cases (3). It has no gender preference and can affect patients from neonatal period to eighth decade of life but mainly among children and young adult patients (4). Importantly, though rare, most cases are at an advanced stage and progress rapidly to death when diagnosis (5). NMC has a tendency to arise from midline anatomical sites, especially the head and neck and the mediastinum (6,7). However, the primary pulmonary NMC is exceptionally rare from existing literature and the diagnosis and differential diagnosis are challenging. So, in the present study, the authors present a case of NMC arising primarily in the lung and aim to emphasize the diagnosis and treatment of this kind of carcinoma. The following case presented by us conforms with the CARE-Guideline (8). We present the following case in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/acr-19-168).
Case presentation
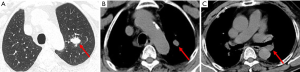
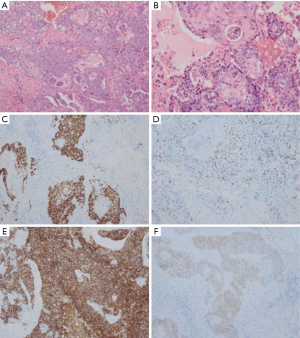
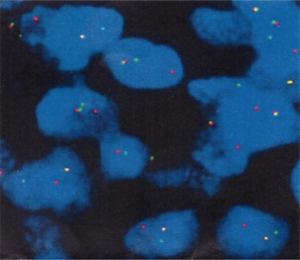
A 69 years old man with no apparent clinical symptoms underwent a physical examination on June 11, 2018. Computed tomography at local hospital demonstrated a mass measuring approximately 1.7 cm × 1.1 cm × 2.5 cm in the apex of left upper lobes as well as the left hilar lymph node (Figure 1), which indicated T3N1M0 IIIa stage disease according to the international standard in TNM staging of pulmonary carcinoma. In addition, there were two subpleural calcified nodules in the anterior segment of the right upper lobe. The patient had a medical history of hypertension and atrial fibrillation for 1 year. Because this patient was in a good general condition and there was no evidence of metastatic disease, he underwent the left upper lobectomy plus regional lymphadenectomy with curative intent. A mass measuring 2.5 cm × 1.5 cm in the apical segment of the left upper lobe can be seen during the surgery. In addition, there was metastatic lymph nodes abutting bronchus confirmed by postoperative pathological diagnosis. After a genetic test using tissues was conducted, an EGFR mutation and the amplification of ALK, KRAS, BRAF, ERBB2, ROS1, NRAS etc. were not detected at that time. An initial diagnosis of neuroendocrine carcinoma with adenocarcinoma was rendered by local hospital and this patient was referred to our center for further management on July 25, 2018. After reviewing the case by our pathologists, the patient was suspected as NMC. A histological examination of surgical specimens revealed a poorly differentiated neoplasm sharing some histopathologic features with squamous cell carcinomas. The tumor cells showed proliferation of nest-like distribution accompanied by focal abrupt keratinization without the gradual differentiation of the squamous epithelial layer and surrounded by fibrous tissue hyperplasia and inflammatory infiltration in different degree. Immunohistochemical staining was diffusely positive for p63 and CD56 and was partially positive for CK56, Ki-67/MIB-1 (+30%) and PCK (Figure 2), but negative for PDL1 (22C3), P40, Syna, CaA, CK7, NapsinA, TTF-1. The reliability of the initial diagnosis of neuroendocrine carcinoma with adenocarcinoma was doubted by our pathologist, because the histopathologic features and immunohistochemical staining did not support it. There was also a possibility of poorly differentiated squamous cell carcinoma, but the patient was a non-smoker and his disease progressed rapidly. Therefore, the fluorescence in situhybridization (FISH) analysis was performed using NUTM1 dual color break apart probe (ZytoLight® SPEC NUTM1 Dual Color Break Apart Probe, ZytovisionGmbH, Germany) on paraffin-embedded sections of resected specimen and the result was positive, which indicated that the percentage of translocation cells was 65% (positive criteria was over 15%) (Figure 3). Given the histopathologic features, immunohistochemistry along with the result of FISH, the diagnosis of NMC was confirmed.
Considering the poor prognosis of NMC and postoperative high-risk recurrence factors, this patient was given further adjuvant chemotherapy of etoposide combined with cisplatin plus bevacizumab (Avastin™), specifically etoposide 130 mg d1, 100 mg d2–5; cisplatin 50 mg d1; 40 mg d2–3, bevacizumab 500 mg d1, 21 days repeated from August 24, 2018 to October 13, 2018. CT of the chest performed after two cycles of combined chemotherapy showed no tumor recurrence or metastasis. Molecular analysis with next-generation sequencing (NGS) were conducted on September 17, 2018 in postoperative tissue and 5 somatic variants were detected by the NGS genetic testing panel (OncoScreen Plus™, Burning Rock Company, China) covering 520 genes including whole exon regions of 312 genes and hotspot mutation regions (exons, introns, and promoter regions) of 208 genes. The results are summarized in Table 1. In this specimen, the tumor mutational burden (TMB) was 1.6 mut/Mb and microsatellite instable (MSI) was microsatellite stable. However, this patient suspended treatment because of acute heart failure on the fourth day of the third cycle of chemotherapy. Later, from November 3, 2018, he accepted etoposide combined with carboplatin plus bevacizumab (etoposide 110 mg d1–2, 100 mg d3–5; carboplatin 340 mg d1; bevacizumab 500 mg d1) in the fourth cycle. On April 26, 2019, the CT of chest indicated no recurrence or metastasis and this patient remains disease-free at 10 months. The timeline was shown in Figure 4. Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.

Full table
Discussion
NMC is an orphan but aggressive disease. Till now, there has been less than 50 cases arising in lung were reported to our best of knowledge in English literature (10). Most patients were at an advanced stage at diagnosis and the 1-, 2- and 5-year overall survival (OS) was 21.81%, 7.27% and 0%, respectively. However, patients with metastatic disease and tumor size more than 5 cm (largest tumor dimension) had significantly poorer OS. So, the present case may have a relatively good prognosis for its non-metastatic disease and small tumor size. Interestingly, NMC is a young age carcinoma, because the median age at diagnosis was only 23 years old for the whole NMC patients as reported (10). It is caused by the rearrangement of chromosome involving the NUT gene, instead of being associated with hundreds of mutations and other environmental factors, which may arise over a period of many years (4). However, in the present study, our case was diagnosed at 69 years old with no symptoms and metastasis. This phenomenon did make some difficulty for the initial diagnosis.
The diagnosis of NMC depends on the demonstration of NUT gene rearrangement or misexpression. There are several methods available for the diagnosis of NMC, such as immunohistochemistry, karyotype, FISH (3), RT-PCR (11) and NGS (12). Because NMC is rare, these methods are not routinely applied to every patient with carcinoma, leading to the misdiagnosis or late diagnosis of this disease.
Till now, there has been no standard therapy for NMC. Surgery is still the primary treatment for NMC if patients can be diagnosed at the early stage. NMC is often initially responsive to chemotherapy and radiation, but it invariably recurs rapidly and does not respond to subsequent therapeutic interventions (13). However, Filippakopoulos et al. found that selective inhibition of BET bromodomains could be effective for NMC (14), and following a novel oral BET inhibitor, OTX015/MK-8628, is currently in clinical development for targeting BRD2/3/4/T, and two NMC patients have responded rapidly to this inhibitor (15). As it is mentioned above, we assess two biomarkers of immunotherapy for this patient. The immunohistochemical staining of PDL1 was negative and the TMB was low expression, which suggest that he might not benefit from immunotherapy.
Stefano et al. (16) firstly reported that metastatic NMC patients carried other somatic mutations including deleted in colorectal cancer (DDC), mixed lineage leukemia protein 3, and splicing factor 3B subunit genes in NMC cells. In the present case, we conduct the NGS for the patient. Interestingly, 5 variants including CCND1, DDR2, CSF1R, DAXX, and RUNX1 were found.
The cyclin D1 (CCND1) gene is located on Chromosome 11q13, the amplification of which can be detected in different kinds of human malignancies. CCND1 encodes cyclin D1 that regulates cell cycle by forming active complexes with either CDK4 or CDK6, which in turn phosphorylate the retinoblastoma protein (Rb) and drive G1 to S phase progression (9). The dysregulation of CCND1 may alter cell cycle progression and contribute to tumorigenesis. Several clinical trials had confirmed CDK4/6 related inhibitors may be sensitive to CCND1-amplified tumors (17). If CCND1 was identified to play a role in NMC, new therapeutic inhibitors targeting CDK4/CDK6 should be considered.
The discoidin domain receptor 2 (DDR2) gene is localized in chromosome 1q23 which is a member of the discoidin domain receptor subclass of the receptor tyrosine kinase (RTKs) protein family. It is known that DDR2 alterations lead to more aggressive phenotypes of cancer (18). Several clinical activity and cases suggest that patients with advanced non-small-cell lung cancer and a DDR2 mutation responded to dasatinib treatment (19-21). However, p.Lys427Glu is not located in functional domain of DDR2 protein, so the efficacy of dasatinib is uncertain for this patient. In addition, immunotherapy in patients with DDR2 mutation is also in debate. MinYuen et al. (22) identified that in patients with urothelial cancer, the effective rate of immunotherapy in patients with deleterious DDR mutation was the higher compared with DDR alterations of unknown significance, but the effective rate was the lowest in patient with wild-type DDR genes, which indicating immune checkpoint inhibitors might be the potential therapy for NMC patients with DDR2 alterations.
A novel colony-stimulating factor 1 receptor (CSF1R) missense mutation, c.2486A>C p.Asp829Ala, was identified in the patient. It is the first time to report this mutation in a malignant tumor according to the TCGA pan-cancer database. It is localized in chromosome 5q32. Mutations in this gene have been associated with an autosomal dominant disease called hereditary diffuse leukoencephalopathy with spheroids and with a predisposition to myeloid malignancy (23). In case of CSF1R mutation involvement in NMC, theoretically, corresponding tyrosine kinase inhibitors can be taken into account.
The overexpression of death domain-associated protein (DAXX) is a common feature in diverse cancers, which correlates with tumorigenesis, disease progression and treatment resistance (24). Additionally, immunohistochemistry analysis of tumor specimens has documented increased DAXX expression levels in several cancer types including prostate, ovarian, oral squamous cell carcinoma, and lung cancer (25). Further studies will be required to clarify roles of DAXX in lung cancer tumorigenesis and to explore the appropriate treatment strategy.
RUNX1 gene is located on chromosome 21 which participates in regulating the differentiation and maturation of hematopoietic stem cells and plays a key role in embryonic development and adult hematopoiesis. However, the overexpressed RUNX proteins is not only associated with leukemia development, but also with solid tumor formation in the skin, lungs, intestines and breasts (26). If the involvement of RUNX1 in NMC was confirmed, there would be more treatment options for this patient.
The strengths of this case are that we demonstrated a rare primary lung NMC with detailed process of diagnosis, treatment and gene mutations which may contribute to the more therapy options for this disease. However, we do not have the opportunity to find out the exact rearrangement of NUTM1 gene fusion. Furthermore, more cases and studies are required to clarify and verify the roles of these gene mutations in NMC.
Conclusions
Primary pulmonary NMC is a rare and aggressive disease with poor prognosis. Till now, there has been less than 50 case arising in lung were reported to our best of knowledge in English literature and this is the first case report of pulmonary NUT carcinomas with DDR2, CCND1, CSF1R, RUNX1 and DAXX somatic mutations, it is aimed at suggesting characteristic diagnostic features and possible therapeutic approaches towards this aggressive neoplasm. However, further clinical trials need to confirm the role of these variants in NMC development and develop potential therapy for NMC patients.
Acknowledgments
Funding: This work was supported by National Key Research and Development Program of China (No. 2018YFC1311400/2018YFC1311402); National Natural Science Foundation of China (grants 81672982).
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/acr-19-168
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/acr-19-168). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work inensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- French C. NUT midline carcinoma. Nat Rev Cancer 2014;14:149-50. [Crossref] [PubMed]
- Kubonishi I, Takehara N, Iwata J, et al. Novelt (15; 19) (q15; p13) chromosome abnormality in a thymic carcinoma. Cancer Res 1991;51:3327-28. [PubMed]
- French CA, Ramirez CL, Kolmakova J, et al. BRD-NUT oncoproteins: A family of closely related nuclear proteins that block epithelial differentiation and maintain the growth of carcinoma cells. Oncogene 2008;27:2237-42. [Crossref] [PubMed]
- French CA. Pathogenesis of NUT midline carcinoma. Annu Rev Pathol 2012;7:247-65. [Crossref] [PubMed]
- Bauer DE, Mitchell CM, Strait KM, et al. Clinicopathologic features and long-term outcomes of NUT midline carcinoma. Clin Cancer Res 2012;18:5773-9. [Crossref] [PubMed]
- Stelow EB, Bellizzi AM, Taneja K, et al. NUT rearrangement in undifferentiated carcinomas of the upper aerodigestive tract. Am J Surg Pathol 2008;32:828-34. [Crossref] [PubMed]
- Evans AG, French CA, Cameron MJ, et al. Pathologic characteristics of NUT midline carcinoma arising in the mediastinum. Am J Surg Pathol 2012;36:1222-7. [Crossref] [PubMed]
- Riley DS, Barber MS, Kienle GS, et al. CARE 2013 Explanations and Elaborations: Reporting Guidelines for Case Reports. J Clin Epidemiol 2017;89:218-35. [Crossref] [PubMed]
- Kato JY, Sherr CJ. Inhibition of granulocyte differentiation by G1 cyclins D2 and D3 but not D1. Proc Natl Acad Sci U S A 1993;90:11513-7. [Crossref] [PubMed]
- Giridhar P, Mallick S, Kashyap L, et al. Patterns of care and impact of prognostic factors in the outcome of NUT midline carcinoma: a systematic review and individual patient data analysis of 119 cases. European Archives of Oto-Rhino-Laryngology 2018;275:815-21. [Crossref] [PubMed]
- Engleson J, Soller M, Panagopoulos I, et al. Midline carcinomawitht (15;19) and BRD4-NUT fusion oncogene in a 30-year-old female with response to docetaxel and radiotherapy. BMC Cancer 2006;6:69. [Crossref] [PubMed]
- Mao N, Liao ZL, Wu JW, et al. Diagnosis of NUT carcinoma of lung origin by next-generation sequencing: case report and review of the literature. Cancer Biol Ther 2019;20:150-6. [Crossref] [PubMed]
- Mertens F, Wiebe T, Adlercreutz C, et al. Successful treatment of a child with t (15;19)-positive tumor. Pediatr Blood Cancer 2007;49:1015-7. [Crossref] [PubMed]
- Filippakopoulos P, Qi J, Picaud S, et al. Selective inhibition of BET bromodomains. Nature 2010;468:1067-73. [Crossref] [PubMed]
- Stathis A, Zucca E, Bekradda M, et al. Clinical response of carcinomas harboring the BRD4-NUT oncoprotein to the targeted bromodomain inhibitor otx015/mk-8628. Cancer Discov 2016;6:492-500. [Crossref] [PubMed]
- Cavalieri S, Stathis A, Fabbri A, et al. Uncommon somatic mutations in metastatic NUT midline carcinoma. Tumori 2017;103:e5-8. [Crossref] [PubMed]
- Suh JH, Johnson A, Albacker L, et al. Comprehensive Genomic Profiling Facilitates Implementation of the National Comprehensive Cancer Network Guidelines for Lung Cancer Biomarker Testing and Identifies Patients Who May Benefit From Enrollment in Mechanism-Driven Clinical Trials. Oncologist 2016;21:684-91. [Crossref] [PubMed]
- Valiathan RR, Marco M, Leitinger B, et al. Discoidin domain receptor tyrosine kinases: new players in cancer progression. Cancer Metastasis Rev 2012;31:295-321. [Crossref] [PubMed]
- Haura EB, Tanvetyanon T, Chiappori A, et al. Phase I/II study of the Src inhibitor dasatinib in combination with erlotinib in advanced non-small-cell lung cancer. J Clin Oncol 2010;28:1387-94. [Crossref] [PubMed]
- Johnson FM, Bekele BN, Feng L, et al. Phase II study of dasatinib in patients with advanced non-small-cell lung cancer. J Clin Oncol 2010;28:4609-15. [Crossref] [PubMed]
- Tu MM, Lee FY, Jones RT, et al. Targeting DDR2 enhances tumor response to anti–PD-1 immunotherapy. Science Advances 2019;5:eaav2437. [Crossref] [PubMed]
- Teo MY, Seier K, Ostrovnaya I, et al. Alterations in DNA Damage Response and Repair Genes as Potential Marker of Clinical Benefit From PD-1/PD-L1 Blockade in Advanced Urothelial Cancers. J Clin Oncol 2018;36:1685-94. [Crossref] [PubMed]
- Rademakers R, Baker M, Nicholson AM, et al. Mutations in the colony stimulating factor 1 receptor (CSF1R) gene cause hereditary diffuse leukoencephalopathy with spheroids. Nat Genet 2011;44:200-5. [Crossref] [PubMed]
- Michaelson J S, Bader D, Kuo F, et al. Loss of Daxx, a promiscuously interacting protein, results in extensive apoptosis in early mouse development. Genes Dev 1999;13:1918-23. [Crossref] [PubMed]
- Mahmud I, Liao D. DAXX in cancer: phenomena, processes, mechanisms and regulation. Nucleic Acids Res 2019;47:7734-52. [Crossref] [PubMed]
- Taniuchi I, Osato M, Ito Y. Runx1: no longer just for leukemia. EMBO J 2012;31:4098-9. [Crossref] [PubMed]
Cite this article as: Liu Y, Li YY, Ke XX, Lu Y. The primary pulmonary NUT carcinomas and some uncommon somatic mutations identified by next-generation sequencing: a case report. AME Case Rep 2020;4:24.



