
Acute pseudogout presenting as an exception to Musculoskeletal Infection Society criteria in total knee arthroplasty: a case report
Introduction
Pseudogout is a subtype of crystalline arthropathy involving deposition of calcium pyrophosphate dihydrate (CPPD) crystals and can present with an erythematous, hot, swollen mono-articular arthritis similar in manifestation to acute septic arthritis. (1) Although crystal deposition with subsequent pain predominantly occurs in a native knee, case literature nonetheless exists which document rare incidents of pseudogout in the setting of total knee arthroplasty (TKA) (2-11). Despite established knowledge of pseudogout prevalence in the setting of total joint arthroplasty, guidelines to-date have required operative irrigation and debridement followed by gold-standard intraoperative cultures to rule out diagnosis of joint co-infection (12).
Clinically, accurate and early determination of PJI remains paramount. The Musculoskeletal Infection Society (MSIS) has published (and subsequently revised) a series of major and minor criteria for accurate diagnosis of prosthetic joint infection, with positive identification considered with presence of one major criterion or a combined weighted score of greater than or equal to 6 points based on six minor criteria (13). Non-invasive analysis of biomarkers available from fluid aspirate samples has more recently been proposed to assess the likelihood of a joint being septic. The most prominent example is the Synovasure® alpha-defensin assay with documented sensitivities and specificities above 95% (14-16). A landmark Level I study independent of assay developers cited 97% sensitivity and specificity, positive predictive value at 88%, and 99% negative predictive value (14). Alpha defensins are small peptides released by neutrophils in response to bacterial infection and detection of their expression by the alpha defensin assay is therefore not affected by prior antibiotic administration or coexisting inflammatory conditions (15,17,18). Noted false positives encompassed cases of metallosis and a solitary false negative assay was found in a patient with a draining sinus that also had multiple operating-room cultures which were negative for 14 days postoperatively. An isolated case report furthermore exists of positive alpha-defensin assay in a case of gout following TKA; however, the patient in question had a history of tophaceous gout (18). This case report acknowledges that there has been previous investigation regarding alpha-defensin testing in cases of pseudogout and the test was appropriately negative (19). Furthermore, this institution has previously reported a case of pseudo-septic arthritis as a late consequence of hyaluronic acid injections, which was confirmed negative with use of this assay (20).
It is the authors’ objective within this report to describe a case of a previously asymptomatic TKA in which the patient presented 13 years post-op with acute pain, swelling, and erythema about the prosthetic knee concerning for infection. Appropriate clinical and laboratory workup were performed in an outpatient setting including recently validated synovial fluid alpha-defensin assay, allowing for infection rule-out in under 24 hours followed by subsequent crystalline arthropathy confirmation without need for any operative irrigation and debridement or intraoperative cultures, as would be required to rule out infection by MSIS criteria. We present the following case in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/acr-20-82).
Case presentation
We present a 78-year-old female with a history of hypertension, hyperlipidemia, gastroesophageal reflux, and cholelithiasis; no history of crystalline arthropathy or immunosuppression was noted. Her index procedure was performed on the left knee in 2004, wherein TKA was completed without complaint and the patient had range of motion from 0–120 degrees of flexion over a decade later. She proceeded to have a right-sided TKA for osteoarthritis in 2017, without complications. Five months after the right TKA, the patient presented with a painful, erythematous, and swollen left knee. The patient denied any preceding illnesses, sick contacts, or contemporaneous dental work. She was afebrile and vital signs were collectively within normal limits. Physical exam was pertinent for left knee erythema, effusion, and limited range of motion secondary to pain. Radiographs failed to demonstrate loosening of components (Figure 1).
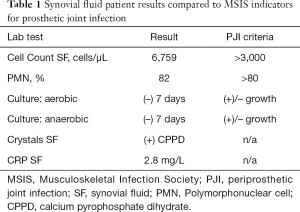
Laboratory evaluation was performed and inflammatory markers at that time were: WBC: 4.8×109 (ref 4.5×109–11.0×109) cells/L, ESR: 38 (ref 0–30) mm/h, and CRP: 58 (ref <3.0) mg/L. Approximately 20cc of white-yellow, murky synovial fluid was aspirated under sterile conditions and sent for intra-articular testing of cell-count, crystals, aerobic and anaerobic cultures, synovial fluid CRP, and Synovasure® alpha-defensin assay; the patient was sent home with instructions to return in the event of persistent fever or worsening pain. According to the newest 2018 evidence based MSIS criteria, this patient evaluated positively for PJI by the following: CRP (2 points), ESR (1 point), synovial fluid polymorphonuclear (PMN) percentage (2 points), and synovial fluid white blood cell (WBC) count (3 points) for a total of 8 points (13). Comprehensive intra-articular panel results were subsequently obtained (Table 1).
Full table
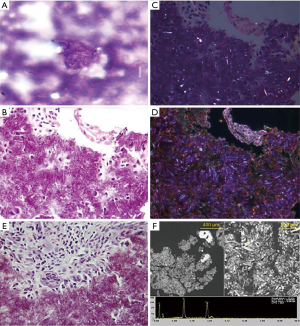
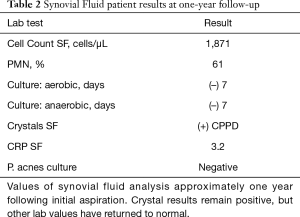
Alpha-defensin data was first reported within 24 hours and indicated that no intraarticular infection was present. Based upon the test’s replicable high sensitivity and specificity (14-16), PJI was ruled-out. Laboratory crystal analysis was reported at 48 hours, noting crystalline deposition consistent with pseudogout. Pseudogout was considered the primary etiology of this patient’s symptomatology given laboratory analysis and observed improvements in her clinical picture after the joint aspiration alongside 1–2 days of low-dose oral NSAIDs. Representative crystal appearances on cyto-histology are shown in Figure 2 (21), and cultures proved negative at seven days for either aerobic or anaerobic colonization. No antibiotics were administered, and the patient was subsequently seen in the office every 2–3 days for 2 weeks in order to evaluate clinical progression. The patient was consequently followed on a symptomatic basis and repeat laboratory analysis was performed on her left knee one year following the described flare, without findings suggestive of PJI (Table 2). At two years post-flare the patient again returned for follow-up and demonstrated excellent bilateral knee range-of-motion without pain, swelling, or any evidence of pseudogout recurrence or infection. We therefore consider implementation of rapid-response Synovasure® alpha-defensin assay to rule out PJI followed by symptom-based pseudogout treatment a success, as invasive irrigation and debridement along with its associated operative and anesthesia risks were avoided with no negative consequences to the patient.
Full table
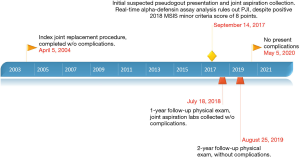
An illustrative timeline of both relevant the patient’s past medical history as well as course of the current case is shown in Figure 3. In the setting of outpatient follow-up, the patient was consulted that her case would be submitted to academic journals for publication and permission was granted.
Written informed consent was obtained from the patient for publication of this Case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Discussion
Following total joint arthroplasty, maintained vigilance for infection remains critical. Given the relative rarity however of crystalline arthropathy following total joint arthroplasty as demonstrated by a paucity in case literature, acute gout and pseudogout diagnosis may be easily missed in practice and mistaken for PJI—a far more serious presentation necessitating invasive procedural confirmation and aggressive management. Under the current MSIS guidelines, the patient’s knee would require irrigation and debridement as well as intraoperative cultures to rule out infection. However, absence of infection was confirmed through application of novel Synovasure® Alpha-defensin lateral flow testing of synovial fluid aspirate and the patient avoided operative treatment, instead being successfully managed medically for an acute pseudogout flare. The authors believe this case to be novel and noteworthy in that lack of infection was confirmed on an outpatient basis, within 24 h, and importantly all whilst avoiding the morbidity of another operative procedure post-TKA. Noteworthy limitations certainly persist in the individual patient sample size reported presently, which reduces surgeons’ ability to generalize management practices in the absence of further confirmatory evidence. Likewise, considering the emergent nature of modern alpha-defensin analysis techniques continued quantification of sensitivity and specificity across wider ranges of patient disease status, demographics, and potential comorbidities may serve to more accurately inform the test’s overall clinical translation as well as any potentially critical exceptions to accuracy in practice. Important follow-up therefore remains critically necessary in this proposed MSIS-criteria based exception to PJI diagnosis, given the aforementioned lack of relevant clinical case literature concurrent to this report presenting findings derived from an individual patient.
Statistical evidence in favor of point-of-care alpha-defensin testing (14-16) for assessment of septic joint likelihood persists robustly in support of greater standardized implementation. Whereas existing MSIS symptom-driven framework of PJI evaluation remains broadly applicable and macroscopically appropriate, highly relevant exceptions exist which although epidemiologically uncommon may considerably alter patient-centered experiences and treatment outcomes. While our patient would also be considered positive for PJI based on both the 2011 MSIS criteria (elevated ESR/CRP, elevated synovial WBC count, elevated synovial fluid PMN%, purulence in the joint) and the 2014 MSIS criteria (elevated ESR/CRP, elevated synovial fluid WBC count, elevated synovial fluid PMN%), the 2018 MSIS criteria includes alpha-defensin, is evidence based, and demonstrates a superior sensitivity of 97.7% compared to the previous 79.3% with specificity of 99.5% (13,22,23). Of additional note stands emerging data suggesting that contrasted to generally excellent empirical alpha-defensin test sensitivity and specificity, MSIS findings strongly correlate to subjective surgeon intent-to-treat with elevated variability skewing towards PJI overestimation (24-26). The patient in this case report had diffuse improvement of symptomatology after aspiration with NSAIDs administration, indicative of correct proactive and non-invasive exclusion of PJI.
To the authors’ knowledge, this is the first report in which suspicion of joint infection was ruled out and pseudogout then identified without need for intraoperative synovial fluid samples, but rather through the use of novel Synovasure® alpha-defensin assay. We propose that clinical diagnosis and management of crystalline arthropathy may be better guided by point-of-care alpha-defensin assay testing, and an important exception to present MSIS criteria for PJI.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist for case reports. Available at http://dx.doi.org/10.21037/acr-20-82
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/acr-20-82). The authors have no conflicts of interest to declare.
Ethical statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this Case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Busso N, Ea HK. The mechanisms of inflammation in gout and pseudogout (CPP-induced arthritis). Reumatismo 2012;63:230-7. [Crossref] [PubMed]
- Escrivá-Fornés M, González-Puig L, Román-Ivorra JA, et al. Pseudogout in a patient with bilateral total knee prosthesis: A challenging diagnosis. Joint Bone Spine 2016;83:463-4. [Crossref] [PubMed]
- Harato K, Yoshida H. Pseudogout in the early postoperative period after total knee arthroplasty. J Arthroplasty 2013;28:374.e9-374.e11. [Crossref] [PubMed]
- Hirose CB, Wright RW. Calcium pyrophosphate dihydrate deposition disease (pseudogout) after total knee arthroplasty. J Arthroplasty 2007;22:273-6. [Crossref] [PubMed]
- Holt G, Vass C, Kumar CS. Acute crystal arthritis mimicking infection after total knee arthroplasty. BMJ 2005;331:1322-3. [Crossref] [PubMed]
- Hunte TC, Bernstein HM, Dickinson GM. Acute crystalline arthritis in an artificial knee. J Clin Rheumatol 2012;18:203-4. [Crossref] [PubMed]
- Levi GS, Sadr K, Scuderi GR. Bilateral pseudogout 8 years after bilateral total knee arthroplasty. Orthop Clin North Am 2012;43:e59-62. [Crossref] [PubMed]
- Sonsale PD, Philipson MR. Pseudogout after total knee arthroplasty. J Arthroplasty 2007;22:271-2. [Crossref] [PubMed]
- Sato R, Nakano S, Takasago T, et al. Chondrogenesis in the synovial tissue is associated with the onset of pseudogout after total knee arthroplasty. Arthroplasty Today 2015;2:101-4. [Crossref] [PubMed]
- Yahia SA, Zeller V, Desplaces N, et al. Crystal-induced arthritis after arthroplasty: 7 cases. Joint Bone Spine 2016;83:559-62. [Crossref] [PubMed]
- Zadaka A, Gioe T, Gertner E. Acute crystal-induced arthritis following arthroplasty. J Knee Surg 2010;23:17-20. [Crossref] [PubMed]
- Cohen-Rosenblum A, Barnett SA, Dewitz R, et al. Prosthetic Septic Arthritis: Etiology, Clinical Aspects, and Management. In: Espinoza LR. editor. Infections and the Rheumatic Diseases. Springer, 2019:63-73.
- Parvizi J, Tan TL, Goswami K, et al. The 2018 Definition of Periprosthetic Hip and Knee Infection: An Evidence-Based and Validated Criteria. J Arthroplasty 2018;33:1309-14.e2. [Crossref] [PubMed]
- Bonanzinga T, Zahar A, Dütsch M, et al. How reliable is the alpha-defensin immunoassay test for diagnosing periprosthetic joint infection? A prospective study. Clin Orthop Relat Res 2017;475:408-15. [Crossref] [PubMed]
- Shahi A, Parvizi J, Kazarian GS, et al. The alpha-defensin test for periprosthetic joint infections is not affected by prior antibiotic administration. Clin Orthop Relat Res 2016;474:1610-5. [Crossref] [PubMed]
- Deirmengian C, Kardos K, Kilmartin P, et al. The alpha-defensin test for periprosthetic joint infection outperforms the leukocyte esterase test strip. Clin Orthop Relat Res 2015;473:198-203. [Crossref] [PubMed]
- Bowdish DM, Davidson DJ, Hancock RE. Immunomodulatory properties of defensins and cathelicidins. Curr Top Microbiol Immunol 2006;306:27-66. [Crossref] [PubMed]
- Partridge DG, Gordon A, Townsend R. False-positive synovial fluid alpha-defensin test in a patient with acute gout affecting a prosthetic knee. Eur J Orthop Surg Traumatol 2017;27:549-51. [Crossref] [PubMed]
- Deirmengian C, Kardos K, Kilmartin P, et al. The Alpha-defensin Test for Periprosthetic Joint Infection Responds to a Wide Spectrum of Organisms. Clin Orthop Relat Res 2015;473:2229-35. [Crossref] [PubMed]
- Korsh JM, Bassett WP, Polakoff DR. Late hemorrhagic pseudoseptic arthritis encountered during total knee arthroplasty due to hyaluronic acid viscosupplementation. Arthroplast Today 2016;2:165-9. [Crossref] [PubMed]
- Naqvi AH, Abraham JL, Kellman RM, et al. Calcium pyrophosphate dihydrate deposition disease (CPPD)/Pseudogout of the temporomandibular joint - FNA findings and microanalysis. Cytojournal 2008;5:8. [Crossref] [PubMed]
- Parvizi J, Zmistowski B, Berbari EF, et al. New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society. Clin Orthop Relat Res 2011;469:2992-4. [Crossref] [PubMed]
- Parvizi J, Gehrke T, Chen AF. Proceedings of the International Consensus on Periprosthetic Joint Infection. Bone Joint J 2013;95-B:1450-2. [Crossref] [PubMed]
- Huard M, Detrembleur C, Poilvache H, et al. Diagnosing chronic periprosthetic joint infection: definition matter. Orthop Proc 2019;101-B:45.
- Huard M, Detrembleur C, Poilvache H, et al. Alpha Defensin: A Diagnostic Accuracy Depending on the Infection Definition Used. J Arthroplasty 2020;35:1355-60. [Crossref] [PubMed]
- Kiran M, Donnelly TD, Armstrong C, et al. Diagnostic utility of fluorodeoxyglucose positron emission tomography in prosthetic joint infection based on MSIS criteria. Bone Joint J 2019;101:910-4. [Crossref] [PubMed]
Cite this article as: Forlizzi JM, Ryan JM, Galow KE, Shang AC, Polakoff DR. Acute pseudogout presenting as an exception to Musculoskeletal Infection Society criteria in total knee arthroplasty: a case report. AME Case Rep 2020;4:21.


