
Development of myocardial infarction and ischemic stroke after acute upper gastrointestinal bleeding
Introduction
Coronary artery disease and stroke are the leading causes of mortality and morbidity worldwide (1). Their incidence and prevalence are steadily rising in China (2). Coronary artery disease refers to narrowing of the coronary arteries secondary to a buildup in the walls of the arteries of plaque, and broken plaque may lead to myocardial infarction (3). Stroke is a neurological deficit attributed to acute focal injury of the central nervous system by a vascular cause, which is divided into ischemic and hemorrhagic stroke (4,5). Common risk factors of coronary artery disease and stroke include smoking, alcohol abuse, hypertension, diabetes mellitus, hyperlipidemia, and psychosocial stress (6,7).
The incidence of acute upper gastrointestinal bleeding is approximately 100/100,000 adults (8). It is a severe clinical symptom (9,10), which leads to hypoperfusion, hemodynamic compromise, ischemic stroke, and myocardial hypoperfusion (11). However, the episodes of myocardial infarction and ischemic stroke secondary to acute upper gastrointestinal bleeding are rare in clinical practice, but the management is often complicated in such patients (12). In this paper, we introduced a patient admitted with acute upper gastrointestinal bleeding who developed both myocardial infarction and ischemic stroke during his hospitalization. We present the following article in accordance with the CARE guideline.
Case presentation
On June 27, 2016, a 73-year-old previously healthy male was admitted to our department due to persistent fatigue and anorexia for more than 1 month and melena for 1 day. He had a long-term history of taking compound aminopyrine phenacetin tablets. He had a long-term history of smoking with 20 cigarettes and drinking with 150 g liquor per day. He denied any history of liver cirrhosis, gastrointestinal bleeding, hypertension, diabetes mellitus, stroke, and coronary heart disease. Before his admission, laboratory tests at his local hospital demonstrated that white blood cell (WBC) was 18.4×109/L (reference range, 3.5–9.5×109/L), neutrophil percentage (GR%) was 71.8% (reference range, 40–75%), hemoglobin concentration (HB) was 68 g/L (reference range, 115–175 g/L), platelet (PLT) was 128×109/L (reference range, 125–350×109/L), blood glucose (BG) was 7.75 mmol/L (reference range, 3.89–6.11 mmol/L), total bilirubin (TBIL) was 6.1 µmol/L (reference range, 5.1–20.0 µmol/L), direct bilirubin (DBIL) was 1.1µmol/L (reference range, 0–6.8 µmol/L), alanine aminotransaminase (ALT) was 22U/L (reference range, 7–50 U/L), aspartate aminotransferase (AST) was 18U/L (reference range, 13–40 U/L), alkaline phosphatase (AKP) was 48 U/L (reference range, 13–150 U/L), γ-glutamyl transpeptidase (γ-GT) was 43 U/L (reference range, 7-60 U/L), and albumin (ALB) was 33 g/L (reference range, 40–55 g/L). The liver function was within the normal range. Abdominal Doppler ultrasound demonstrated heterogeneous echo in liver and gallbladder multiple polyps. Taken the signs of UGIB including fatigue, anorexia, melena, and a decreased HB into consideration, the patient was diagnosed with acute upper gastrointestinal bleeding. Intravenous infusion of esomeprazole was given at his admission.
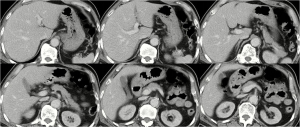
On June 28, 2016, the patient was apathy accompanying with decreased computing capability. Laboratory tests demonstrated that WBC was 22.4×109/L, GR% was 85.6%, HB was 52 g/L, PLT was 86×109/L, BG was 5.79 mmol/L, TBIL was 5.7 µmol/L, DBIL was 2.1 µmol/L, ALT was 13.78 U/L, AST was 12.87 U/L, AKP was 49.49 U/L, γ-GT was 41.63 U/L, ALB was 23.8 g/L, blood urea nitrogen (BUN) was 47.25 mmol/L (reference range, 2.9–8.2 mmol/L), creatinine (Cr) was 267.63 µmol/L (reference range, 44–133 µmol/L), creatine kinase (CK) was 318 U/L (reference range, 38–174 U/L), CK-myocardial band (CK-MB) was 15 U/L (reference range, 0–24 U/L), hypersensitive C-reactive protein (hCRP) was 39.9 mg/L (reference range, 0–3 mg/L), hypersensitive troponin T (hs-TNT) was 0.054 ng/mL (reference range, 0–0.05 ng/mL), prothrombin time (PT) was 14.1 s (reference range, 11.5–14.5 s), and activated partial thromboplastin time (APTT) was 42.6 s (reference range, 28.0–40.0 s). Series of viral hepatitis were negative. Chest X-ray demonstrated enlarged heart and aortic sclerosis. Contrast-enhanced abdominal computed tomography demonstrated suspicion of liver cirrhosis and hepatic S2 segment nodule (Figure 1). Intravenous infusion of cefoperazone sodium sulbactam sodium was additionally given. Isosorbide dinitrate tablets were orally taken, and 1.5 units of red blood cell was transfused.
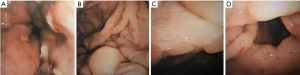
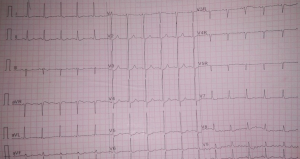
On June 29, 2016, his condition was stable without melena. An upper gastrointestinal endoscopy was performed after a written informed consent from the patient and his relatives was obtained. Upper gastrointestinal endoscopy demonstrated reflux esophagitis and multiple ulcers at the antrum (Figure 2). However, on the evening of June 29, 2016, his condition was suddenly exacerbated presenting with continuous pain under the xiphoid. The 18-lead electrocardiogram demonstrated ST-segment depression in leads V3-V6 and flat T-wave in leads I, II, III, aVL, aVF, V5, and V6 (Figure 3). Laboratory tests demonstrated that WBC was 16.4×109/L, GR% was 86.8%, HB was 58 g/L, PLT was 84×109/L, BG was 5.65 mmol/L, C-reactive protein (CRP) was 113.2 mg/L (reference range, ≤10 mg/L), CK was 1,613 U/L, CK-MB was 32 U/L, hCRP was 85.7mg/L, and hs-TNT was 0.036 ng/mL. Taken the typical signs of myocardial infarction including continuous pain under the xiphoid and an increased hs-TNT into consideration, cardiologist considered that non-ST elevation myocardial infarction wasn’t excluded. Antithrombotic and interventional therapy weren’t recommended under his current condition. Intravenous infusion of isosorbide mononitrate was prescribed. Additionally, 2 units of red blood cell was transfused.
On June 30, 2016, his pain under the xiphoid slightly improved with limb weakness. Laboratory tests demonstrated that WBC was 15.0×109/L, GR% was 82.3%, HB was 66g/L, PLT was 78×109/L, BG was 5.14 mmol/L, CRP was 42.6 mg/L, CK was undetectable, CK-MB was 42U/L, hCRP was 87.9 mg/L, hs-TNT was 0.036 ng/mL, and N-type B-terminal natriuretic peptide (NT-proBNP) was 3,930 pg/mL (reference range, 0–125 pg/mL). Magnetic resonance of brain demonstrated multiple cerebral infarction and brain stem infarction. Taken the signs of cerebral infarction including limb weakness and decreased computing capability into consideration, the patient was diagnosed with ischemic stroke. Torasemide was intravenously injected, and 2 units of red blood cell was transfused.
On July 1, 2016, his condition was temporarily stable without melena anymore. Laboratory tests demonstrated that WBC was 10.5×109/L, GR% was 75.4%, HB was 82 g/L, PLT was 93×109/L, BG was 5.32 mmol/L, TBIL was 7.7 µmol/L, DBIL was 2.8 µmol/L, ALT was 16.84 U/L, AST was 44.25 U/L, AKP was 57.16 U/L, γ-GT was 57.04 U/L, ALB was 26.9 g/L, BUN was 14.49mmol/L, Cr was 141.09 µmol/L, CK was 1153U/L, CK-MB was 25 U/L, hCRP was 80.5 mg/L, and hs-TNT was 0.033 ng/mL. There was a significantly increased HB and decreased CK. Intravenous infusion of cefoperazone sodium sulbactam sodium, torasemide, and esomeprazole were continuously given.
On the morning of July 5, 2016, his condition was suddenly exacerbated again presenting with hematemesis and melena. Immediate laboratory tests demonstrated that WBC was 13.3×109/L, GR% was 62.5%, HB was 79 g/L, PLT was 113×109/L, BG was 11.57 mmol/L, BUN was 12.95 mmol/L, and Cr was 166.40 µmol/L. Intravenous infusion of esomeprazole, somatostatin, and hydroxyethyl starch were given. Thrombin and Yunnan Baiyao were orally taken, and 2 units of red blood cell was transfused.
On the afternoon of July 5, 2016, his condition was stable without gastrointestinal bleeding. But the patient and his relatives decided to discharge because of unaffordable medical expenses. Unfortunately, he died on July 12, 2016.
Discussion
Acute upper gastrointestinal bleeding is a serious complication with a mortality of 3–14% (13). It is more severe in cirrhotic patients than in patients without liver cirrhosis (14). However, this patient did not perform liver biopsy. Only a suspected diagnosis of liver cirrhosis was made. Then, this patient did not have clear sign of portal hypertension, such as splenomegaly and varices. Herein, the potential reason of gastrointestinal bleeding may be gastric ulcer. On the other hand, laboratory tests demonstrated that the levels of BUN and Cr were significantly increased. However, the patient didn’t meet the criteria of hepatorenal syndrome (15). And his renal function was improved after treatment. Herein, the patient should be diagnosed with pre-renal renal hypoperfusion secondary to gastrointestinal bleeding (16). In this case, the possible causes for developing myocardial infarction and ischemic stroke after acute upper gastrointestinal bleeding are as follows. First, hypoperfusion secondary to massive acute gastrointestinal bleeding led to the ischemia of various organs, including heart and brain (11,17). Second, this patient had a long-term history of smoking and alcohol abuse. Smoking is a risk factor of cardiovascular disease, which causes endothelial dysfunction and atherogenesis (18,19). Although 1–2 drinks per day are a negative risk factor of myocardial infarction and ischemic stroke, harmful drinking (consuming ethanol ≥61.0 g per day for men) significantly increases the risk of death from cardiovascular disease (20-22). Third, this patient had an advanced age, which is a major risk factor of cardiovascular disease (23). He was more vulnerable to suffer from severe acute upper gastrointestinal bleeding episode than young adult. Fourth, cardiac embolism was also a risk factor of ischemic stroke (24). This patient was diagnosed as ischemic stroke after a diagnosis of myocardial infraction, therefore myocardial infraction might be a potential cause for ischemic stroke. After that, his magnetic resonance of brain demonstrated multiple cerebral infarction and brain stem infarction.
Acute upper gastrointestinal bleeding, myocardial infarction, and ischemic stroke are all leading causes of death worldwide (25). This patient developed severe upper gastrointestinal bleeding followed by myocardial infarction and ischemic stroke. This represents a clinical challenge, because antithrombotic therapy, which should be employed for myocardial infarction and ischemic stroke, such as aspirin and heparin, is often contraindicated in a patient with acute bleeding, and hemostatic therapy, which is employed for controlling bleeding, may be harmful in a patient with myocardial infarction and ischemic stroke (26,27). This patient refused further treatment and died several days after discharge. The survival is closely associated with age, severity of ischemic events, and treatment (28). This patient could not receive effective antithrombotic and interventional therapy for myocardial infarction and ischemic stroke, thereby leading to an unsatisfactory outcome.
In conclusion, our case reminds the clinicians of a possibility of developing of cardiovascular disease episode after acute upper gastrointestinal bleeding. Further well-designed studies are needed to explore the association between cardiovascular disease and gastrointestinal bleeding and to identify the high-risk population.
Acknowledgments
The authors would like to thank the patient for participating.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/acr-19-198). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The patient in our manuscript died after discharge. The study was conducted in accordance with the Declaration of Helsinki(as revised in 2013). We tried our best to contact with the next of kin of the patient by telephone, but we failed. We just reviewed the case to share a rare clinical experience in this case report.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015 Lancet 2016;388:1459-544. [published correction appears in Lancet. 2017 Jan 7;389(10064):e1]. [Crossref] [PubMed]
- Shen C, Ge J. Epidemic of Cardiovascular Disease in China. Circulation 2018;138:342-4. [Crossref] [PubMed]
- Parmet S, Glass TJ, Glass RM. JAMA patient page. Coronary artery disease. Jama 2004;292:2540. [Crossref] [PubMed]
- Sacco RL, Kasner SE, Broderick JP, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44:2064-89. [Crossref] [PubMed]
- Hankey GJ. Stroke. Lancet 2017;389:641-54. [Crossref] [PubMed]
- O'Donnell MJ, Chin SL, Rangarajan S, et al. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case-control study. Lancet 2016;388:761-75. [Crossref] [PubMed]
- Joseph P, Leong D, McKee M, et al. Reducing the Global Burden of Cardiovascular Disease, Part 1: The Epidemiology and Risk Factors. Circ Res 2017;121:677-94. [Crossref] [PubMed]
- Rockall TA, Logan RF, Devlin HB, et al. Incidence of and mortality from acute upper gastrointestinal haemorrhage in the United Kingdom. Steering Committee and members of the National Audit of Acute Upper Gastrointestinal Haemorrhage. Bmj 1995;311:222-6. [Crossref] [PubMed]
- Zheng K, Yoshida EM, Tacke F, et al. Risk of Stroke in Liver Cirrhosis: A Systematic Review and Meta-Analysis. J Clin Gastroenterol 2020;54:96-105. [Crossref] [PubMed]
- del Olmo JA, Pena A, Serra MA, et al. Predictors of morbidity and mortality after the first episode of upper gastrointestinal bleeding in liver cirrhosis. J Hepatol 2000;32:19-24. [Crossref] [PubMed]
- Cappell MS. Gastrointestinal bleeding associated with myocardial infarction. Gastroenterol Clin North Am 2000;29:423-44. vi. [Crossref] [PubMed]
- Qi X, Qiu J, De Stefano V, et al. Development of acute ischemic stroke in two patients with acute upper gastrointestinal bleeding. AME Med J 2017;2:24. [Crossref]
- van Leerdam ME. Epidemiology of acute upper gastrointestinal bleeding. Best Pract Res Clin Gastroenterol 2008;22:209-24. [Crossref] [PubMed]
- Lisman T, Porte RJ. Rebalanced hemostasis in patients with liver disease: evidence and clinical consequences. Blood 2010;116:878-85. [Crossref] [PubMed]
- Shah N, Silva RG, Kowalski A, et al. Hepatorenal syndrome. Dis Mon 2016;62:364-75. [Crossref] [PubMed]
- Bai Z, Primignani M, Guo X, et al. Incidence and mortality of renal dysfunction in cirrhotic patients with acute gastrointestinal bleeding: a systematic review and meta-analysis. Expert Rev Gastroenterol Hepatol 2019;13:1181-8. [Crossref] [PubMed]
- Caplan LR, Wong KS, Gao S, et al. Is hypoperfusion an important cause of strokes? If so, how? Cerebrovasc Dis 2006;21:145-53. [Crossref] [PubMed]
- Iribarren C, Tekawa IS, Sidney S, et al. Effect of cigar smoking on the risk of cardiovascular disease, chronic obstructive pulmonary disease, and cancer in men. N Engl J Med 1999;340:1773-80. [Crossref] [PubMed]
- Messner B, Bernhard D. Smoking and cardiovascular disease: mechanisms of endothelial dysfunction and early atherogenesis. Arterioscler Thromb Vasc Biol 2014;34:509-15. [Crossref] [PubMed]
- Britton A, McKee M. The relation between alcohol and cardiovascular disease in Eastern Europe: explaining the paradox. J Epidemiol Community Health 2000;54:328-32. [Crossref] [PubMed]
- Liang X, Wang L, Jiang Y, et al. Prevalence of cardiovascular disease riak factors in China: findings from 2010 China chronic disease and risk factor surveillance. Heart 2012;98:E134-5. [Crossref]
- Mukamal KJ, Conigrave KM, Mittleman MA, et al. Roles of drinking pattern and type of alcohol consumed in coronary heart disease in men. N Engl J Med 2003;348:109-18. [Crossref] [PubMed]
- Lakatta EG. Age-associated cardiovascular changes in health: impact on cardiovascular disease in older persons. Heart Fail Rev 2002;7:29-49. [Crossref] [PubMed]
- Kamel H, Healey JS. Cardioembolic Stroke. Circ Res 2017;120:514-26. [Crossref] [PubMed]
- GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015 Lancet 2016;388:1459-544. [published correction appears in Lancet. 2017 Jan 7;389(10064):e1]. [Crossref] [PubMed]
- Leong DP, Joseph PG, McKee M, et al. Reducing the Global Burden of Cardiovascular Disease, Part 2: Prevention and Treatment of Cardiovascular Disease. Circ Res 2017;121:695-710. [Crossref] [PubMed]
- Sverdén E, Markar SR, Agreus L, et al. Acute upper gastrointestinal bleeding. Bmj 2018;363:k4023. [Crossref] [PubMed]
- Norris RM. The natural history of acute myocardial infarction. Heart 2000;83:726-30. [Crossref] [PubMed]
Cite this article as: Zheng K, Xu X, Qi X, Guo X. Development of myocardial infarction and ischemic stroke after acute upper gastrointestinal bleeding. AME Case Rep 2020;4:20.


