
Unique pathological findings of lung adenocarcinoma after unexpected nivolumab treatment, possible different effects on the primary lesion and metastatic lymph nodes: case report
Introduction
Nivolumab is a fully human IgG4 programmed death 1 (PD-1) immune-checkpoint inhibitors that disrupts PD-1-mediated signaling and restores antitumor immunity. The efficacy and survival benefit of nivolumab in advanced non-small cell lung cancer patients have been demonstrated in several clinical studies (1,2). However, there are few opportunities to observe histological changes of tumors after nivolumab treatment because immune-checkpoint inhibitors are considered standard treatment for advanced non-small cell lung cancer patients. Here, we present an extremely rare case with unique pathological findings of lung adenocarcinoma after unexpected preoperative nivolumab treatment that was followed by surgery.
Case presentation
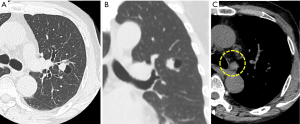
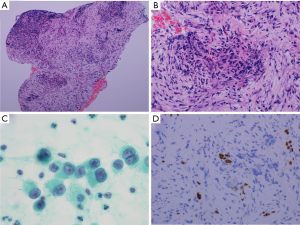
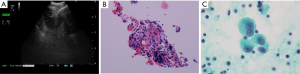
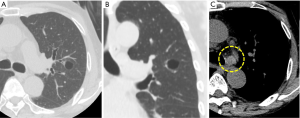
A 69-year-old Japanese man was referred to our department for further examination of a left lung nodule. Non-contrast chest computed tomography (CT) scans revealed a well-defined solid nodular shadow, 1.4 cm × 1.1 cm in size, partially filling an inner cavity of a bulla in the left upper lobe (Figure 1A,B) and an abnormally swollen lymph node in the station #4L, measuring 1.4 cm × 1.3 cm (Figure 1C). We avoided using a contrast agent due to his renal functional impairment. Positron-emission tomography (PET)-CT showed a high fluorodeoxyglucose (FDG) uptake and a standardized uptake value (SUV) max =5.2 in the station #4L lymph node, implying nodal metastasis, but there was no evidence of distant metastases. Transbronchial lung biopsy (TBLB) and transbronchial aspiration cytology (TBAC) for the pulmonary nodule and endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) for the station #4L lymph node were performed, and cancer cells with enlarged nucleoli were detected from the pulmonary nodule (Figure 2A,B,C). Based on the results of immunohistochemical assay of TTF-1 (Figure 2D), we diagnosed the disease as primary lung adenocarcinoma. The same cancer cells were also detected from #4L lymph node (Figure 3A,B,C). Thus, clinical stage of the disease was determined as cT1aN2M0-stage IIIA (TNM 7th). Chemoradiotherapy, the standard treatment for cN2, stage IIIA adenocarcinoma, was considered impossible due to his renal functional impairment. So surgery was the only radical treatment option and therefore planned. However, we required more than two months for the preoperative risk assessment including a detailed examination of cardiac function. A preoperative chest CT scan performed three months after the first scan revealed surprisingly an apparent shrinking of the pulmonary nodule (Figure 4A,B). We suspected the possibility of supplement intake in a covert manner or very unusual natural shrinking. In contrast to the pulmonary nodule, the station #4L lymph node demonstrated no change in size (Figure 4C). We performed a left upper lobectomy and mediastinal lymph node dissection. The postoperative course was uneventful, and the patient was discharged on postoperative day 12.
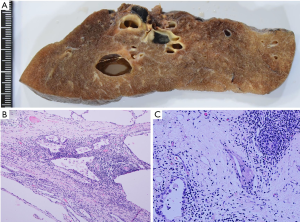
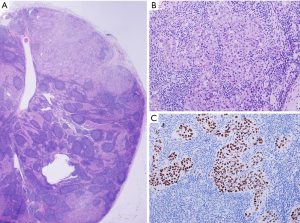
Pathologically, the cystic wall of the lung lesion was lined solely by alveolar epithelial cells, and no adenocarcinoma cells were confirmed. In a thickened part of the cystic wall, a foreign body granuloma composed of foamy macrophages and cholesterol clefts was observed among fibrous tissue, suggesting that tumor cells disappeared after some treatment (Figure 5A,B,C). However, adenocarcinoma cells were confirmed in the station #4L, #10 and #12u lymph nodes (Figure 6A,B,C). The final pathological stage was determined to be pT0N2M0. Epidermal growth factor receptor activating mutations were not detected by the real-time PCR method (Cobas EGFR mutation test, Roche Diagnostics, Tokyo, Japan) of the TBLB specimen. Immunohistochemically, tumor cells in the resected lymph node #4L were negative for anaplastic lymphoma kinase.
Six weeks after surgery, the patient complained of fatigue and drowsiness and finally confessed that he had undergone a preoperative treatment, which he had privately received at a different clinic under his own will. He had been treated with nivolumab 20 mg per body twice, 1 and 2 months before surgery. He was suspected of developing hypothyroidism as a side effect of the nivolumab. Fortunately, his thyroid function immediately improved spontaneously.
Discussion
Nivolumab is a representative immune-checkpoint inhibitor used for the treatment of malignant melanoma and is known to be effective for the treatment of several solid malignancies. As a treatment for lung cancer, it was first approved for advanced squamous cell lung cancer as a second-line treatment, and its indication is extending to other non-small cell lung cancers (1,2). Nivolumab and other immune-checkpoint inhibitors are now certainly changing the treatment strategies of lung cancer. Concurrently, we find new knowledge obtained about various kinds of immune-related adverse events, like thyroid hypofunction and type 1 diabetes mellitus which are not seen in classical cytotoxic agents or other targeted therapeutics (3). However, little is known about histological changes of lung cancer after immune-checkpoint inhibitor treatment. For example, pseudoprogression is reported as characteristic phenomenon after immunomodulation therapy (4). Although it is thought to be initial enlargement of tumor shadow caused by transient immune cell infiltration, its mechanism and histological change remain unclear.
Histological changes of lung cancer after neoadjuvant chemotherapy have been documented with regard to tumor regression (5). We observed similar pathological findings in the primary lesion of our case, such as foamy macrophages and cholesterol clefts, and the adenocarcinoma cells vanished. However, no such changes were seen, and tumor cells remained in the lymph nodes. We additionally performed programmed death-ligand 1 (PD-L1) immunohistochemistry assay. PD-L1 expression was observed only 5% of tumor cells in TBLB specimen and 1% in the resected lymph node #4L. There is a discrepancy between the pathological good response of the primary lesion and the low PD-L1 expression for TBLB specimen. Possible reasons for the discrepancy are as follows. One is underestimation of PD-L1 expression due to small number of tumor cells in TBLB specimen. The other reason is intratumoral heterogeneity of the tumor cells in the primary lesion. In the latter case, only tumor cells with low PD-L1 expression might possibly metastasize to the lymph nodes, or some kind of transformation might have occurred to the tumor cells during the metastasizing process. Actually, Uruga et al. reported that up to 38% of Stage II and III lung adenocarcinoma cases showed discrepant PD-L1 expression between the primary tumor and the metastatic lymph node, raising the possibility of intratumoral heterogeneity of PD-L1 expression (6). In addition, the low dosage of nivolumab might also have some effect on the outcome. The patient received a total of only 40 mg, 20 mg twice, which was approximately one sixth of the recommended dose.
We also thought about PD-L2 expression which was reported as another ligand and possible predictor of the clinical response to anti-PD-1 targeted therapies (7). But PD-L2 expression was not observed in the resected lymph node #4L by immunohistochemistry assay.
This is the detailed reported case of lung adenocarcinoma that disappeared after preoperative immune-checkpoint inhibitor treatment. Although limited to the primary tumor, its effectiveness was histologically confirmed. There may be a possibility that immune-checkpoint inhibitors will be commonly used for neoadjuvant treatment in certain selected patients (8). On this occasion, we must manage several severe adverse effects. We can predict fatal patient situations if operative stress is added to immune-related adverse events. In the present case, we were not aware of the preoperative nivolumab treatment and the patient developed hypothyroidism postoperatively. Although his health was improving, his condition might have been more critical if he was treated with the usual, much higher dose.
We described a rare case with unique pathological findings of lung adenocarcinoma after unexpected preoperative nivolumab treatment in which the adenocarcinoma disappeared and the lymph node metastases did not. This case report may provide a clue to the future development of induction therapy using nivolumab and surgery.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
References
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the Immune-Related Adverse Effects of Immune Checkpoint Inhibitors: A Review. JAMA Oncol 2016;2:1346-53. [Crossref] [PubMed]
- Tanizaki J, Hayashi H, Kimura M, et al. Report of two cases of pseudoprogression in patients with non-small cell lung cancer treated with nivolumab-including histological analysis of one case after tumor regression. Lung Cancer 2016;102:44-8. [Crossref] [PubMed]
- Junker K, Thomas M, Schulmann K, et al. Tumour regression in non-small-cell lung cancer following neoadjuvant therapy. Histological assessment. J Cancer Res Clin Oncol 1997;123:469-77. [Crossref] [PubMed]
- Uruga H, Bozkurtlar E, Huynh TG, et al. Programmed Cell Death Ligand (PD-L1) Expression in Stage II and III Lung Adenocarcinomas and Nodal Metastases. J Thorac Oncol 2017;12:458-66. [Crossref] [PubMed]
- Yearley JH, Gibson C, Yu N, et al. PD-L2 Expression in Human Tumors: Relevance to Anti-PD-1 Therapy in Cancer. Clin Cancer Res 2017;23:3158-67. [Crossref] [PubMed]
- Forde PM, Chaft JE, Smith KN, et al. Neoadjuvant PD-1 Blockade in Resectable Lung Cancer. N Engl J Med 2018;378:1976-86. [Crossref] [PubMed]
Cite this article as: Nakao M, Noma D, Ichinose J, Matsuura Y, Mun M, Nakagawa K, Shigematsu Y, Ninomiya H, Ishikawa Y, Okumura S. Unique pathological findings of lung adenocarcinoma after unexpected nivolumab treatment, possible different effects on the primary lesion and metastatic lymph nodes: case report. AME Case Rep 2019;3:45.


