
Late-onset severe pneumonitis under osimertinib
Introduction
Osimertinib is approved for 1st line treatment in EGFR-mutated non-small cell lung cancer (NSCLC) and T790M-positive NSCLC after previous tyrosine kinase inhibitor (TKI) treatment. Osimertinib is a third-generation TKI and in general very well tolerated. However, pneumonitis is a rare and possibly fatal side effect that requires discontinuation of TKI treatment. We report of a case with late onset severe pneumonitis under osimertinib and briefly discuss further tumor treatment options.
Case presentation
A 61 years old male never-smoker with stage IVa (cT3cN3cM1a) NSCLC presented to the emergency department with four days of increasing shortness of breath and new onset of night sweats. NSCLC had been diagnosed 20 months prior and as the tumor harbored a common EGFR Exon 21 mutation (L858R c.2573T>G) initially treated with afatinib. After 10 months of afatinib treatment the patient developed progressive disease and repeat biopsy of the mediastinal lymph nodes showed a T790M mutation. Second line treatment with osimertinib 80 mg daily was initiated in September 2017. He had initially experienced a partial remission on osimertinib, and repeat imaging after 2 and 5 months of treatment showed no evidence of progressive disease. The medical history was otherwise unremarkable, with no history of interstitial lung disease, asthma, COPD or pulmonary embolism.
At the time of emergency presentation the patient had been on treatment with osimertinib for 9 months and had been tolerating the treatment well, with no evidence of gastrointestinal, cutaneous or cardiac side-effects. In the emergency room increasing dyspnea and new onset of night sweats for four days were noted. Physical exam revealed a patient in respiratory distress without central or peripheral cyanosis. Vital signs showed: heart rate 95 bpm, blood pressure 164/88 mmHg, O2 saturation 92% breathing room air, breath rate 16 per minute, temperature of 37.0 °C. There were bilateral inspiratory crackles over the lower lung fields. Initial capillary blood gases on room air showed respiratory insufficiency (pH 7.46, pO2 48.3 mmHg, pCO2 38.0 mmHg) in combination with decreased TLC, VC and FEV1. Blood work showed slightly elevated inflammatory parameters [CRP 4.0 mg/dL (standard <0.5 mg/dL), PCT <0.1 ng/mL, leucocytes 7.53 G/L]. D-Dimer was not elevated. Computer tomography of the chest showed multifocal, diffuse bipulmonary opacities predominantly in the upper lobes (Figure 1A).
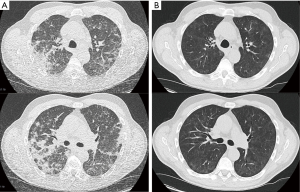
Supplemental oxygen was administered, a bronchoscopy with bronchoalveolar lavage (BAL) was performed and empiric broad spectrum antibiotics were initiated. Differential cytology of the BAL revealed a lymphocytic eosinophilic alveolitis (27.4% lymphocytes, 14.5% eosinophils). Bacterial cultures of BAL fluid showed small amounts of pseudomonas aeruginosa. Viral and fungal infections were not identified.
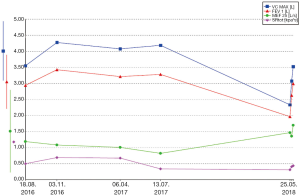
A diagnosis of grade 3 osimertinib-induced predominantly eosinophilic pneumonitis was made, however an osimertinib induced organizing pneumonia seems also feasible. Osimertinib was discontinued and immunosuppressive treatment with prednisolone 100 mg daily was administered for 3 days and then tapered to 1 mg/kg bodyweight (60 mg/daily). Repeat CT scan and lung function test were performed after 9 days of prednisolone treatment. The CT scan revealed nearly complete resolution of the opacities with minimal residual subpleural opacity (Figure 1B). The lung function recovered to 91% of the previous FEV1, to 84% of the previous VC and to 96% of the previous TLC values (Figure 2).
Discussion
The third-generation tyrosine kinase inhibitor (TKI) osimertinib was first approved for treatment of advanced adenocarcinoma of the lung with T790M-resistance mutation after previous TKI treatment. More recent data show that osimertinib is also effective in the first line treatment of NSCLC harboring common activating EGFR mutations (1). Pneumonitis is a known rare side-effect of osimertinib (1,2) and is generally observed in the first three months after initiation of TKI-treatment (2-5). In our case predominantly eosinophilic pneumonitis developed acutely after 9 months of osimertinib treatment. Eosinophilic pneumonitis can occur under many drugs (6) and has been reported for osimertinib (7). Physicians should be aware of this possible and late complication and undertake diagnostic measures to differentiate pneumonitis from bacterial, viral or fungal infection as well as from tumor progression. In addition to supportive measures and discontinuation of osimertinib, immunosuppressive therapy should be initiated with systemic steroids.
There is little data to guide further tumor therapy following pneumonitis under osimertinib in T790M-positive NSCLC. Case reports describe successful re-challenge with osimertinib parallel to systemic prednisolone (4,5,8,9), however only two cases were described as pneumonitis grade 3 or 4. Due to the severity of the pneumonitis and the lack of evidence of disease progression we decided against an early re-challenge with osimertinib and will continue to monitor the patient closely. In general, further systemic treatment should be considered in patients with progressive disease and good general condition. Options include chemotherapy, immune therapy and combined treatments. However, addition of immunotherapy might result in increasing occurrence of pneumonitis for patients with TKI-treatment (3,10). Moreover immunotherapy shows low response rates in EGFR-mutated NSCLC and is currently not recommended as a treatment option (11-13).
Conclusions
Interstitial lung disease, such as predominantly eosinophilic pneumonitis can occur even after long time treatment with osimertinib and is a possible fatal side effect. TKI treatment has to be stopped and administration of steroids considered. Tumor therapy options following pneumonitis include re-challenge, chemotherapy and immunotherapy or combined treatments. However, more data is needed for therapy recommendation.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:113-25. [Crossref] [PubMed]
- Jänne PA, Yang JC, Kim DW, et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med 2015;372:1689-99. [Crossref] [PubMed]
- Mamesaya N, Kenmotsu H, Katsumata M, et al. Osimertinib-induced interstitial lung disease after treatment with anti-PD1 antibody. Invest New Drugs 2017;35:105-7. [Crossref] [PubMed]
- Kiriu T, Tamura D, Tachihara M, et al. Successful Osimertinib Rechallenge with Steroid Therapy after Osimertinib-induced Interstitial Lung Disease. Intern Med 2018;57:91-5. [Crossref] [PubMed]
- Miyauchi E, Ichinose M, Inoue A. Successful Osimertinib Rechallenge in a Patient with T790M-Mutant Non-Small Cell Lung Cancer after Osimertinib-Induced Interstitial Lung Disease. J Thorac Oncol 2017;12:e59-61. [Crossref] [PubMed]
- Bartal C, Sagy I, Barski L. Drug-induced eosinophilic pneumonia: A review of 196 case reports. Medicine (Baltimore) 2018;97:e9688. [Crossref] [PubMed]
- Tachi H, Shiozawa T, Sakai C, et al. Osimertinib-Induced Interstitial Lung Disease Presenting as Eosinophilic Pneumonia. J Thorac Oncol 2017;12:e118-20. [Crossref] [PubMed]
- Nagasaka M, Gadgeel SM. Retreatment With Osimertinib Following Pneumonitis. Clin Lung Cancer 2018;19:e53-5. [Crossref] [PubMed]
- Satoh S, Shiroyama T, Tamiya M, et al. Successful osimertinib rechallenge after osimertinib-induced pneumonitis in a patient with lung adenocarcinoma. Respir Med Case Rep 2017;23:68-70. [Crossref] [PubMed]
- Ahn MJ, Sun JM, Lee SH, et al. EGFR TKI combination with immunotherapy in non-small cell lung cancer. Expert Opin Drug Saf 2017;16:465-9. [Crossref] [PubMed]
- Hsu WH, Yang JC, Mok TS, et al. Overview of current systemic management of EGFR-mutant NSCLC. Ann Oncol 2018;29:i3-9. [Crossref] [PubMed]
- Gainor JF, Shaw AT, Sequist LV, et al. EGFR Mutations and ALK Rearrangements Are Associated with Low Response Rates to PD-1 Pathway Blockade in Non-Small Cell Lung Cancer: A Retrospective Analysis. Clin Cancer Res 2016;22:4585-93. [Crossref] [PubMed]
- Ettinger DS, Wood DE, Aisner DL, et al. Non-Small Cell Lung Cancer, Version 5.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2017;15:504-35. [Crossref] [PubMed]
Cite this article as: Syunyaeva Z, Berghof K, Kauffmann-Guerrero D, Götschke J, Tufman A, Kahnert K. Late-onset severe pneumonitis under osimertinib. AME Case Rep 2019;3:39.


