
Idiopathic stroke after syndromic and neuromuscular scoliosis surgery: a case report and literature review
Introduction
Children with syndromic and neuromuscular scoliosis undergoing corrective spinal surgery have higher complication rates than those with idiopathic scoliosis (1,2). This is thought to be due to comorbidities, the burden of chronic disease, and severity of presentation. Stroke after scoliosis surgery is rare, occurring in less than 0.57% of those with neuromuscular disease (NMD) (3). However, it is a disproportionately morbid outcome, accounting for 7.6% of deaths associated with scoliosis surgery in the Scoliosis Research Society (SRS) morbidity and mortality database (4).
We present a case of a 15-year-old boy with neurofibromatosis type I (NF-1) and dystrophic thoracolumbar kyphosis who underwent revision circumferential fusion for a non-union and implant fracture. The case was complicated by a cerebrovascular accident (CVA) on postoperative day (POD) one with no identifiable cause. Recovery was complete by six weeks except for mild dysphagia and dysarthria. Our purpose is to raise awareness of this rare but devastating complication in order to allow for appropriate counseling, preoperative evaluation, and expeditious diagnosis and intervention.
Case presentation
History
A 5-year-old boy with NF-1 presented to another facility with paraplegia. Workup demonstrated thoracolumbar kyphosis associated with a circumferential neurofibroma eroding T12 (Figure 1). He underwent emergent decompressive laminectomy without fusion or instrumentation, followed by bracing. His postoperative exam included bilateral lower limb spasticity and 4/5 muscle function, such that he used a walker outside the home. Because of his age and progressive kyphosis, he underwent multiple growing rod lengthenings and revision instrumentations until fifteen years of age, when he presented with rod fracture at the site of laminectomy resulting in focal kyphosis. The most recent spinal operation consisted of posterior revision instrumentation and rib strut grafting across the laminectomy, followed by anterior fusion and instrumentation. The circumferential neurofibroma necessitated separate approaches and the assistance of an abdominal surgeon to partially resect and reflect it off the anterior spine. Neither patient nor family have a history of thromboembolic events. At baseline, he has lower limb spasticity, is independent in activities of daily living (ADLs), able to ambulate independently at home, uses a walker or scooter out of the home, and wears ankle-foot orthotics.
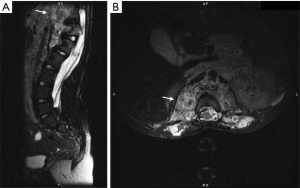
Examination and imaging
The patient presented with a new prominence over his spine at the thoracolumbar junction, without overlying soft tissue in jeopardy. Röntgenogrammes showed bilateral rod fracture and ipsilevel focal kyphosis with apex left scoliosis (Figure 2). Physical examination of the lower limbs showed no change in baseline neural function: 4/5 hip flexion, and 4/5 ankle dorsiflexion and plantarflexion, 5/5 other lower limb muscle groups, hamstring spasticity producing knee flexion contractures, bilateral ankle clonus, intact light touch but reduced sharp/dull sensation, and normal bowel and bladder function.
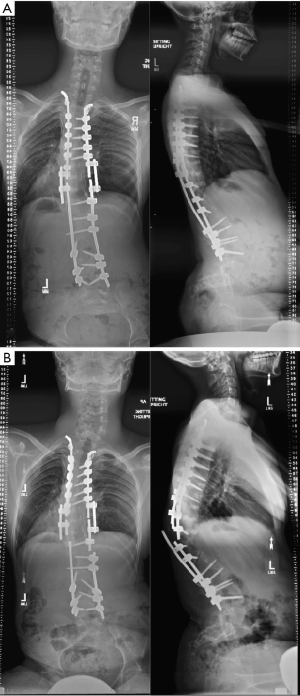
Operation
The patient underwent revision posterior T6-L4 instrumentation and fusion (Figure 3). This was complicated by a 1 cm dural tear at the apex of kyphosis at T12-L1, in the region of the index laminectomy and where there was extensive neurofibroma enveloping the spine. An estimated 20 ml of cerebrospinal fluid leaked out. The tear was repaired with 4-0 silk suture, TISSEEL, and muscle overlay. A Valsalva manoeuvre demonstrated no CSF leak after the repair. A plastic surgeon performed a complex wound closure and reconstruction with bilateral paraspinous muscle flaps.
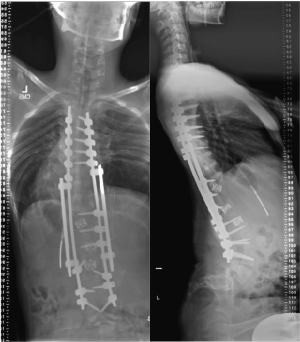
This was followed by an anterior approach for T11-L2 instrumentation and fusion. A right lateral tenth rib thoracoabdominal approach exposed the kyphosis between T11 and L2. We were assisted by an abdominal surgeon who partially resected and reflected the large neurofibroma that had eroded the vertebral bodies from T11-L2. Due to the complexity of the neurofibroma and previous abdominal surgery, we were unable to find a local arterial pedicle for an autogenous vascularized fibula strut-graft. Remnant intervertebral discs were excised from T11-L2. Pyramesh® cages (Medtronic Sofamor Danek USA, Inc) filled with autogenous costal graft were placed. Bone morphogenetic protein was not used given age and risk of malignant transformation.
Total surgical time was 12 hours. Neurophysiologic monitoring was stable at baseline. Estimated blood loss was 750 mL. The patient received three units of packed red blood cells and three liters of fluids. Blood pressure was stable (mean arterial pressures 60–70 s). Deep and superficial drains were placed posteriorly. A chest tube as well as a superficial drain were placed anteriorly.
Postoperative course
Postoperative day zero and one evaluation demonstrated baseline neural function. The patient then experienced an acute change in mental status on POD1 during which his eyes suddenly rolled back and he became dysarthric and somnolent. He had a National Institutes of Health Stroke Scale (NIHSS) score of 22 indicating severe stroke, with decline in mental status, expressive aphasia, right horizontal gaze restriction, right hemiplegia, and decreased sensation to noxious stimuli on the right. Immediate noncontrast head computed tomography (CT) revealed a large, expansile region of hypo-attenuation in the right cerebellum and obstructive hydrocephalus from associated mass effect due to upper transtentorial and downward tonsillar herniation (Figure 4). The neurological surgeons performed emergent occipital craniotomy for decompression and external ventricular drain (EVD) placement.
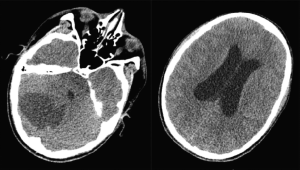
Following craniotomy, the patient remained intubated for management of intracranial pressure (ICP) and EVD. Neuroprotective measures included steroids, ICP goal <20 mmHg, sodium goal 140–150 mEq/L, normoglycemia, normothermia, and elevated head of bed. Magnetic resonance imaging (MRI) on POD4 revealed a right cerebellar infarct involving the right superior and posterior inferior cerebellar artery (PICA) territories, as well as smaller posterior cerebral artery infarct involving left thalamus and right posterior splenium. The right PICA tapered abruptly beyond its midsegment, likely due to occlusion. Neural function improved such that by POD5 he was alert and able to follow one step commands for thumb and finger movements bilaterally. The patient was extubated on POD7. At that time, he was oriented, following commands, and able to activate all extremity muscle groups. The right side was more affected (e.g., finger tapping was quicker on the left). MRI of the brain on POD9 demonstrated reduction of swelling, improvement of mass effect on the fourth ventricle with no brainstem displacement, improvement in hydrocephalus, and absence of new infarct or hemorrhage. EVD was clamped on POD10, and removed on POD12 after a stable repeat MRI.
A stroke workup was performed. There was elevation of anti-cardiolipin IgG (aCL) and lipoprotein(a) (LPA), but normal Factor V Leiden, fasting homocysteine, beta-2-glycoprotein antibody IgG and IgM, lupus anticoagulant, and prothrombin 20210 mutation. Doppler ultrasound of the upper and lower extremities showed no evidence of deep vein thrombosis. Echocardiographic bubble study showed no evidence of patent foramen ovale or other cause of right-to-left shunt. MRI with angiography (MRA) of the head and neck was negative for vascular dissection or other anomaly. Röntgenography of spine showed acceptable alignment and implants. The patient was anticoagulated with 81 mg aspirin daily. At discharge on POD24 the patient had regained normal mental status, extraocular movements, fine coordination (including rapid finger tapping and no dysmetria), speech and sensation, in addition to demonstrating baseline motor function and spasticity.
Discussion
The patient’s noncontrast head CT revealed cerebellar herniation and hydrocephalus. Since clinically apparent cerebellar edema after infarct develops over 24–48 hours, the patient’s ischemic event likely occurred intraoperatively. The distribution of the stroke was geographic, consistent with a thromboembolic phenomenon. However, workup demonstrated no right-to-left cardiac shunt and normal cervical vasculature. Furthermore, the patient’s hematologic workup was negative aside from elevated aCL and LPA, which were likely reactive. The patient also had no personal or family history of thromboembolic events despite an extensive surgical history. Overall, this suggests an underlying primary hypercoagulable state was unlikely to be the cause of his stroke.
The association between NMD and hypercoagulability is unclear. Hypercoagulability has been linked to Duchenne muscular dystrophy (DMD), with elevated levels of thrombin-antithrombin complex and plasmin-α2 plasmin inhibitor complex compared with controls (5). The authors noted that the abnormalities persisted without regard to age, respiratory function, cardiac function, or ADLs, suggesting that muscular dystrophy itself is a risk factor for thrombosis (5). However, in two studies of stroke in DMD, coagulation profiles were normal, implicating cardiomyopathy as a potential cause (6,7). No studies have described an association between NF-1 and hypercoagulability.
Certain NMDs are associated with cardiac manifestations that can increase the risk for systemic thromboemboli (8). One case report describes a paradoxical air embolism through a patent foramen ovale (PFO) as the cause of a previously well 46-year-old patient’s stroke after scoliosis surgery (9). Another study found that patients undergoing scoliosis surgery who had the highest numbers of transcranial Doppler signals for cerebral microemboli also had PFOs (10). Other NMDs, including DMD, Becker muscular dystrophy, Friedreich ataxia, and myotonic dystrophy type 1, may present with dilated or hypertrophic cardiomyopathy, as well as arrhythmias such as atrial fibrillation or flutter, that can contribute to stroke risk (8). Impaired systolic function leading to stasis increases the likelihood of intracardiac thrombus and subsequent embolization (8). At this time, data on anticoagulation in children with NMDs are lacking, but thrombosis prophylaxis should be considered in those with aforementioned arrhythmias (8). In our patient, echocardiography and electrocardiography ruled out structural heart disease or conduction abnormality.
NF-1 is associated with cerebrovascular abnormalities that may cause stroke. Cerebral arteriopathy is present in 2–6% of children with NF-1 (11-13). These abnormalities include stenoses, ectatic vessels, aneurysm, and moyamoya (13). The majority of patients are asymptomatic at diagnosis, but up to 47% go on to develop focal neurologic deficits months to years later (12). The onset of symptoms is usually gradual, with the patient experiencing transient ischemic attacks before more permanent deficits arise (11). Our patient’s MRA only showed abrupt tapering most consistent with occlusion from thromboembolism. Cerebral vasculature was otherwise normal (Figure 5).
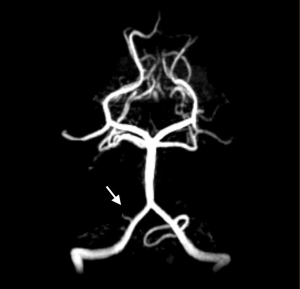
Patient positioning may be difficult in patients with non-idiopathic scoliosis due to associated contractures, deformity, or prior operation. Compression of vertebral arteries from prone positioning and excessive neck movement have been demonstrated as causes of cerebral ischemia (14,15). In one case report, a 69-year-old patient undergoing lumbar laminectomy and fusion had acute bilateral cerebellar ischemia on CT two hours postoperatively, presumed to be due to excessive neck rotation and extension (14). In another report, a 66-year-old obese patient suffered bilateral cortical blindness immediately after lumbar surgery, likely due to difficulties in positioning of the head and body (15). In our patient, MRA showed normal cervical vasculature, ruling out occlusion or dissection secondary to physical vascular damage during positioning.
Dural tear and CSF leak may also cause stroke. The incidence of durotomy during thoracolumbar surgery is estimated at 5.1% (16). This risk is three times higher in revision surgery, and is associated with a 7.7% rate of neurologic complication (17). Multiple case reports have implicated durotomy and subsequent CSF leak in stroke after spine surgery (18,19). All of these strokes included a component of intracranial hemorrhage. One possible mechanism is that loss of CSF leads to relative intracranial hypotension and rupture of venous sinuses (19). Space occupying hematoma may mechanically lead to herniation and vascular occlusion. Loss of CSF may lead to caudal displacement of the brain, kinking arteries to cause infarction (18). Our patient had an MRA that demonstrated an abrupt tapering of the right PICA that was beyond its mid-segment (Figure 5). Unilateral occlusion at this anatomic position is unlikely to be due to compression from caudal displacement of the brain. Furthermore, all other vessels were normal, and there were no signs of intracranial hemorrhage, meaning dural tear was unlikely to be the cause of stroke in our patient.
Pediatric stroke following scoliosis surgery is a rare but devastating complication. Compared with hemispheric stroke, cerebellar stroke may be difficult to diagnose because findings tend to be subtle. Furthermore, the etiology may not always be identified, as the International Pediatric Stroke Study found no identifiable risk factors in 9% of cases (20). Careful attention to speech, gait, coordination, and eye movements is necessary. Frequent serial neurologic and mental status examinations in the peri- and postoperative period is essential in order to detect the risk of stroke in advance, particularly in patients with non-idiopathic scoliosis or abnormal baseline neurological function. Recovery from anesthesia may mask findings in the postoperative period. Although the cause may remain obscure, diagnosis of stroke should be made expeditiously in order to optimize treatment and outcomes. Furthermore, while there is no evidence that syndromic or neuromuscular scoliosis directly increase the risk of post-operative stroke, the related comorbidities associated with these conditions may correlate with an increased risk. As such, careful patient selection and surgical planning is imperative in order to minimize complications and accurately convey risk as part of the informed consent process.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
References
- Levy BJ, Schulz JF, Fornari ED, et al. Complications associated with surgical repair of syndromic scoliosis. Scoliosis 2015;10:14. [Crossref] [PubMed]
- Reames DL, Smith JS, Fu KMG, et al. Complications in the Surgical Treatment of 19,360 Cases of Pediatric Scoliosis. Spine 2011;36:1484-91. [Crossref] [PubMed]
- Mohamad F, Parent S, Pawelek J, et al. Perioperative complications after surgical correction in neuromuscular scoliosis. J Pediatr Orthop 2007;27:392-7. [Crossref] [PubMed]
- Smith JS, Saulle D, Chen CJ, et al. Rates and causes of mortality associated with spine surgery based on 108,419 procedures: a review of the Scoliosis Research Society Morbidity and Mortality Database. Spine 2012;37:1975-82. [Crossref] [PubMed]
- Saito Y, Komiya T, Kawai M. Hypercoagulable state in Duchenne muscular dystrophy. Rinsho Shinkeigaku 1997;37:374-8. [PubMed]
- Hanajima R, Kawai M. Incidence of cerebral infarction in Duchenne muscular dystrophy. Muscle Nerve 1996;19:928. [PubMed]
- Winterholler M, Holländer C, Kerling F, et al. Stroke in Duchenne Muscular Dystrophy: A Retrospective Longitudinal Study in 54 Patients. Stroke 2016;47:2123-6. [Crossref] [PubMed]
- Feingold B, Mahle WT, Auerbach S, et al. Management of Cardiac Involvement Associated With Neuromuscular Diseases: A Scientific Statement From the American Heart Association. Circulation 2017;136:e200-31. [Crossref] [PubMed]
- Pham Dang C, Péréon Y, Champin P, et al. Paradoxical air embolism from patent foramen ovale in scoliosis surgery. Spine 2002;27:E291-5. [Crossref] [PubMed]
- Rodriguez RA, Letts M, Jarvis J, et al. Cerebral microembolization during pediatric scoliosis surgery: a transcranial doppler study. J Pediatr Orthop 2001;21:532-6. [Crossref] [PubMed]
- Cairns AG, North KN. Cerebrovascular dysplasia in neurofibromatosis type 1. J Neurol Neurosurg Psychiatry 2008;79:1165-70. [Crossref] [PubMed]
- Rea D, Brandsema JF, Armstrong D, et al. Cerebral arteriopathy in children with neurofibromatosis type 1. Pediatrics 2009;124:e476-83. [Crossref] [PubMed]
- Rosser TL, Vezina G, Packer RJ. Cerebrovascular abnormalities in a population of children with neurofibromatosis type 1. Neurology 2005;64:553-5. [Crossref] [PubMed]
- Harman F, Yayci F, Deren S, et al. Acute cerebellar ischemia after lumbar spinal surgery: a rare clinical entity. J Anesth 2012;26:947-8. [Crossref] [PubMed]
- Huber JF, Grob D. Bilateral cortical blindness after lumbar spine surgery. A case report. Spine 1998;23:1807-9. [Crossref] [PubMed]
- Garreau de Loubresse C. Neurological risks in scheduled spinal surgery. Orthop Traumatol Surg Res 2014;100:S85-90. [Crossref] [PubMed]
- McMahon P, Dididze M, Levi AD. Incidental durotomy after spinal surgery: a prospective study in an academic institution. J Neurosurg Spine 2012;17:30-6. [Crossref] [PubMed]
- Andrews RT, Koci TM. Cerebellar herniation and infarction as a complication of an occult postoperative lumbar dural defect. AJNR Am J Neuroradiol 1995;16:1312-5. [PubMed]
- Khalatbari MR, Khalatbari I, Moharamzad Y. Intracranial hemorrhage following lumbar spine surgery. Eur Spine J 2012;21:2091-6. [Crossref] [PubMed]
- Mackay MT, Wiznitzer M, Benedict SL, et al. Arterial ischemic stroke risk factors: the International Pediatric Stroke Study. Ann Neurol 2011;69:130-40. [Crossref] [PubMed]
Cite this article as: Nguyen T, Khanna K, Gornitzky AL, Diab M. Idiopathic stroke after syndromic and neuromuscular scoliosis surgery: a case report and literature review. AME Case Rep 2019;3:28.


