
Dysphagia following lumbar spine surgery in the setting of undiagnosed DISH of the cervical spine: a case report
Introduction
Dysphagia is a broadly described condition with an incidence that continues to rise as a larger portion of the population reaches an advanced age (1,2). Oropharyngeal dysphagia leads to an impairment of swallowing initiation that may also result from physiologic aging or be influenced by a variety of neuromuscular conditions (2). Esophageal motility also changes drastically with the aging process, further contributing to multifactorial difficulty with the safe and effective consumption of liquids and solids (3,4).
Anterior cervical hyperostosis (ACH) refers to excessive osteophyte formation and bony overgrowth of the ventral cervical spine. This may result from diffuse idiopathic skeletal hyperostosis (DISH), degenerative spondylosis, post-traumatic change or adjacent segment disease following spinal fusion; these processes have been described in several case reports and case series (5-13).
The pre-vertebral fascia forms the posterior border of the laryngopharynx, creating an intimate anatomic relationship from C2 to C5. The pharyngoesophageal junction is located at the C5-C6 disc space. The esophagus then extends directly anterior to the anterior longitudinal ligament at the midpoint of the vertebral bodies; its course progresses anterior to the aorta at T8, then to the diaphragmatic hiatus at T10 and ends at the gastroesophageal junction at approximately T11 (14). The esophagus is anatomically tethered at the level of the cricoid and the diaphragm, resulting in close adherence to much of the subaxial spine. From C5 to C7, the distance from the esophagus to the cervical spine ranges from just 1 to 3 mm in normal adults (15).
Overgrowth of bone along the anterior spine can lead to dysphagia via several proposed mechanisms. Direct compression of the posterior laryngopharynx or esophagus may reduce lumen patency, limit mobility, or impair the neuromuscular mechanism required for swallowing; motion of soft tissue over a focal area of compression may also result in inflammation and edema (16-18).
Senile ankylosing hyperostosis of the spine was first described in 1950 by Forestier, with clear radiographic and clinical distinctions from ankylosing spondylitis (19). It was presented as a novel pathology without peripheral joint involvement and which featured thick bulging bony protuberances from the vertebral bodies most notable on lateral radiographs. This condition was subsequently known as Forestier’s disease until 1975. Resnick suggested the name DISH in a series describing the extra-spinal manifestations of the condition including involvement of the pelvis, foot and elbow (20).
Prevalence of DISH is poorly defined, though it is understood to generally increase with age with described values ranging from 2.5% to 33.3% in elderly populations (21,22). It typically presents with pain and stiffness and may be radiographically diagnosed by “flowing ossification” along the anterolateral aspects of multiple vertebral segments with the absence of significant degenerative changes or ankylosis of the facet or sacroiliac joints. The thoracic spine is by far the most common site of involvement, followed by the lumbar and cervical regions, respectively (22). Within the cervical spine, manifestations are most common at the C4-C7 levels (10).
We present a case of an elderly patient undergoing lumbar spine decompression and fusion for spinal stenosis in the setting of lumbar DISH. His postoperative course was complicated by significant dysphagia likely caused in part by DISH of the cervical spine that was undiagnosed preoperatively. We have recommended routine pre-operative imaging of the cervical spine for all patients who may be suspected to have cervical DISH or may otherwise be predisposed to multi-factorial dysphagia following surgery.
Case presentation
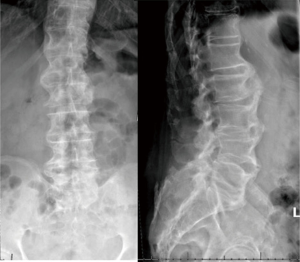
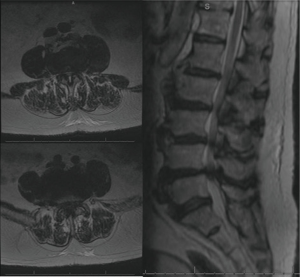
The patient is an 83-year-old male who suffered from low back and bilateral leg pain for many years. It progressed steadily to limit his ability to walk more than one half block at a time despite adhering to physical therapy and several trials of anti-inflammatory medication. He also described numbness and subjective weakness of the legs. Physical exam revealed 4/5 strength in all major muscle groups of the bilateral lower extremities as well as some subjectively diminished sensation in the bilateral feet. Initial radiographs revealed diffuse degenerative changes most significant at the anterior vertebral bodies where osteophytic overgrowth that had progressed to near autofusion (Figure 1). MRI demonstrated multiple degenerative lumbar disks and severe stenosis from L3-S1 (Figure 2). A diagnosis of lumbar spinal stenosis in the setting of DISH resulting in severe neurogenic claudication was made, and after appropriate counseling he elected to proceed with L3-S1 posterior decompression.
Preoperative workup was notable only for hypertension, chronic kidney disease and gastroesophageal reflux. He had no history of diabetes, a noted risk factor for both dysphagia and DISH (23). On the day of surgery, he underwent uneventful endotracheal intubation, with a total anesthesia time of 5 hours and 42 minutes and estimated blood loss of 518 mL. Extubation was uneventful and postoperatively he was admitted to the surgical ICU for monitoring; he received 1 unit of blood and remained clinically stable. He tolerated sips of water several hours after surgery.
On the morning of postoperative day (POD) 1 the patient complained of difficulty swallowing liquids and oral medications. An evaluation was conducted by a speech-language pathologist (SLP); and recommendations were for NPO and prompt administration of an alternate source of nutrition. A Dobhoff tube was placed for administration of medications and enteral feeding. Upon direct questioning at this time, he did describe occasional episodes of coughing when swallowing liquids over the course of the several months preceding surgery. He had not sought any medical attention for this complaint. Daily SLP evaluation was remained consistent with generalized oropharyngeal weakness leading to signs and symptoms of aspiration.
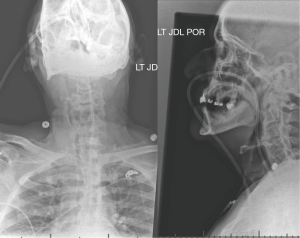
On POD 2 an ENT consultation was obtained; laryngoscopy revealed a unilateral hypomobile vocal cord though with maintained full glottic closure. No clear etiology was determined. On POD 4 a cervical spine radiograph was obtained to further investigate etiologies of his symptoms (Figure 3). At this time, significant anterior hyperostosis was noted with diffuse nonmarginal osteophytes and general maintenance of disc space height; a diagnosis of cervical DISH was made.
On POD 5 a barium swallow study was recommended due to persistent delayed swallow response; this study demonstrated persistent pharyngeal weakness. On POD 7, PO intake was gradually advanced which progressed to pureed liquids by POD 9. The patient was discharged on POD 9 to an inpatient rehabilitation center where his recovery progressed. Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Discussion
Post-operative dysphagia is a well-established complication of cervical spine surgery, particularly from an anterior approach, while lumbar spine surgery is not a known direct risk factor (24-27). Smith-Hammond et al conducted a prospective cohort study to determine the incidence and risk factors for postoperative dysphagia after cervical and lumbar spine surgery (28). There was subjective and objective evidence of dysphagia in a significant proportion of patients with indications for cervical spine surgery, though not in those undergoing lumbar surgery. The incidence of acute and clinically significant postoperative dysphagia was 50% in patients undergoing anterior cervical spine surgery, 20% in those undergoing posterior cervical surgery, and insignificant in posterior lumbar surgery controls. Age was the only significant risk factor. The etiologies of acute dysphagia following anterior cervical spine surgery has been well documented, including direct effect of intraoperative retraction pressure as well as mechanical effect of hardware on the posterior laryngopharynx or esophagus (29).
The direct correlation between incidence of postoperative dysphagia and duration of endotracheal intubation has been described extensively in a wide variety of patient populations, though most studies have evaluated cohorts with a total intubation time exceeding 48 hours (30,31). Increased subjective symptoms of dysphagia post-operatively in patients undergoing thyroid surgery with endotracheal intubation versus laryngeal mask airway (LMA) has been described (32). Proposed mechanisms include direct irritation of the submucosa of the vocal chords and trachea, though surgical manipulation of nearby structures may have also played a significant role given this specific procedure. There is a general lack of literature describing the incidence of dysphagia after routine endotracheal intubation for elective surgery lasting several hours or less.
We describe the case of an elderly male with significant postoperative dysphagia following lumbar spine surgery. At the time of intubation and extubation there were no apparent difficulties, and total duration of intubation was far less than the range considered prolonged and typically discussed in the context of post-extubation dysphagia. Globally depressed neuromuscular function after general anesthesia as well as pharyngeal irritation from intubation and/or extubation is proposed exacerbating factors of what was likely undiagnosed subclinical dysphagia preoperatively. This preexisting condition was likely caused, at least in large part, by anterior osteophytes and hyperostosis of the cervical spine resulting from DISH, of which the treatment team was unaware before surgery. Knowledge of this likely predisposition to post-operative dysphagia could have allowed for early involvement of SLP services post-operatively. While corticosteroid administration has been shown to be of significant benefit in acute dysphagia following anterior cervical spine surgery, the lack of known local causal factors in this case of a lumbar surgery would have precluded this intervention. Similarly, no surgical intervention was indicated for the treatment of post-operative dysphagia in the setting of cervical DISH after this lumbar procedure due to the absence of confirmation that anterior hyperostosis was the sole casual factor, as well as the patients improvement with supportive treatment.
Three studies evaluating whole-spine imaging of patients with diagnoses of DISH have characterized the relative incidences of location of disease; among these studies there is universal support of the understanding that thoracic involvement is much more prevalent than cervical or lumbar (22,33,34). Upon evaluation of 178 whole-spine radiographs of patients diagnosed with DISH at any location, none were found to have isolated cervical or lumbar disease. The 88.7% of patients had disease isolated to the thoracic spine, and 11.3% had diffuse type, with disease in at least 2 of the 3 spinal segments (cervical, thoracic, lumbar) (33). Kim et al. found that for patients diagnosed with DISH at any location, the prevalence of cervical and lumbar disease was present in 7.9% and 7.3%, respectively (34) . The incidence of cervical involvement in the setting of known lumbar disease has not been described.
The authors suggest that routine preoperative cervical spine radiographs be carried out for all elderly patients with a known diagnosis of DISH in other spinal regions. Lumbar disease in isolation or even in addition to disease elsewhere can be considered relatively atypical, and as such there may be additional reason to suspect cervical involvement. With a relatively non-invasive, convenient and inexpensive diagnostic test, a significant risk factor for post-operative dysphagia may be identified. Increased awareness of this risk enables the maximization of peri-operative measures and preparation to reduce the significant morbidity associated with this complication. Further characterization of the incidence of cervical disease given a diagnosis of DISH involving the lumbar spine may help to further specify for which patients this recommendation should apply.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Bloem BR, Lagaay AM, van Beek W, et al. Prevalence of subjective dysphagia in community residents aged over 87. BMJ 1990;300:721-2. [Crossref] [PubMed]
- Achem SR, Devault KR. Dysphagia in aging. J Clin Gastroenterol 2005;39:357-71. [Crossref] [PubMed]
- Tibbling L, Gustafsson B. Dysphagia and its consequences in the elderly. Dysphagia 1991;6:200-2. [Crossref] [PubMed]
- Talley NJ, Weaver AL, Zinsmeister AR, et al. Onset and disappearance of gastrointestinal symptoms and functional gastrointestinal disorders. Am J Epidemiol 1992;136:165-77. [Crossref] [PubMed]
- Calisaneller T, Ozdemir O, Tosun E, et al. Dysphagia due to diffuse idiopathic skeletal hyperostosis. Acta Neurochir (Wien) 2005;147:1203-6. [Crossref] [PubMed]
- Hughes TA, Wiles CM, Lawrie BW, et al. Case report: dysphagia and sleep apnoea associated with cervical osteophytes due to diffuse idiopathic skeletal hyperostosis (DISH). J Neurol Neurosurg Psychiatry 1994;57:384. [Crossref] [PubMed]
- Kissel P, Youmans JR. Posttraumatic anterior cervical osteophyte and dysphagia: surgical report and literature review. J Spinal Disord 1992;5:104-7. [Crossref] [PubMed]
- McGarrah PD, Teller D. Posttraumatic cervical osteophytosis causing progressive dysphagia. South Med J 1997;90:858-60. [Crossref] [PubMed]
- Nelson RS, Urquhart AC, Faciszewski T. Diffuse idiopathic skeletal hyperostosis: a rare cause of dysphagia, airway obstruction, and dysphonia. J Am Coll Surg 2006;202:938-42. [Crossref] [PubMed]
- Ortega-Martínez M, Cabezudo JM, Gomez-Perals LF, et al. Anterior cervical osteophyte causing dysphagia as a complication of laminectomy. Br J Neurosurg 2005;19:174-8. [Crossref] [PubMed]
- Srinivas P, George J. Cervical osteoarthropathy: an unusual cause of dysphagia. Age Ageing 1999;28:321-2. [Crossref] [PubMed]
- Ozgocmen S, Kiris A, Kocakoc E, et al. Osteophyte-induced dysphagia: report of three cases. Joint Bone Spine 2002;69:226-9. [Crossref] [PubMed]
- Oppenlander ME, Orringer DA. Dysphagia due to anterior cervical hyperosteophytosis. Surg Neurol 2009;72:266-70. [Crossref] [PubMed]
- Kuo B, Urma D. Esophagus—anatomy and development. In: Goyal RK, Shaker R. editors. GI Motility Online. New York, NY: Nature Publishing Group, 2006.
- Rhyne AL, Spector LR, Schmidt GL, et al. Anatomic mapping and evaluation of the esophagus in relation to the cervical vertebral body. Eur Spine J 2007;16:1267-72. [Crossref] [PubMed]
- Seidler TO, Pèrez Àlvarez JC, Wonneberger K, et al. Dysphagia caused by ventral osteophytes of the cervical spine: clinical and radiographic findings. Eur Arch Otorhinolaryngol 2009;266:285-91. [Crossref] [PubMed]
- Brandenberg G, Leibrock LG. Dysphagia and dysphonia secondary to anterior cervical osteophytes. Neurosurgery 1986;18:90-3. [Crossref] [PubMed]
- Kmucha ST, Cravens RB Jr. DISH syndrome and its role in dysphagia. Otolaryngol Head Neck Surg 1994;110:431-6. [Crossref] [PubMed]
- Forestier J, Rotes-querol J. Senile ankylosing hyperostosis of the spine. Ann Rheum Dis 1950;9:321-30. [Crossref] [PubMed]
- Resnick D, Shaul SR, Robins JM. Diffuse idiopathic skeletal hyperostosis (DISH): Forestier's disease with extraspinal manifestations. Radiology 1975;115:513-24. [Crossref] [PubMed]
- Belanger TA, Rowe DE. Diffuse idiopathic skeletal hyperostosis: musculoskeletal manifestations. J Am Acad Orthop Surg 2001;9:258-67. [Crossref] [PubMed]
- Toyoda H, Terai H, Yamada K, et al. Prevalence of Diffuse Idiopathic Skeletal Hyperostosis in Patients with Spinal Disorders. Asian Spine J 2017;11:63-70. [Crossref] [PubMed]
- Mader R, Lavi I. Diabetes mellitus and hypertension as risk factors for early diffuse idiopathic skeletal hyperostosis (DISH). Osteoarthr Cartil 2009;17:825-8. [Crossref] [PubMed]
- Baron EM, Soliman AM, Gaughan JP, et al. Dysphagia, hoarseness, and unilateral true vocal cord impairment following anterior cervical diskectomy and fusion. Ann Otol Rhinol Laryngol 2003;112:921-6. [Crossref] [PubMed]
- Bazaz R, Lee MJ, Yoo JU. Incidence of dysphagia after anterior cervical spine surgery: a prospective study. Spine 2002;27:2453-8. [Crossref] [PubMed]
- Yue WM, Brodner W, Highland TR. Persistent swallowing and voice problems after anterior cervical discectomy and fusion with allograft and plating: a 5 to 11 year follow-up study. Eur Spine J 2005;14:677-82. [Crossref] [PubMed]
- Anderson KK, Arnold PM. Oropharyngeal Dysphagia after anterior cervical spine surgery: a review. Global Spine J 2013;3:273-86. [Crossref] [PubMed]
- Smith-Hammond CA, New KC, Pietrobon R, et al. Prospective analysis of incidence and risk factors of dysphagia in spine surgery patients: comparison of anterior cervical, posterior cervical, and lumbar procedures. Spine 2004;29:1441-6. [Crossref] [PubMed]
- Chen D, Shao MM, Wang XY, et al. Current strategies of reduce the rate of dysphagia and dysphonia after anterior cervical spine surgery and role of corticosteroids. Ann Transl Med 2018;6:S99. [Crossref] [PubMed]
- Skoretz SA, Flowers HL, Martino R. The incidence of dysphagia following endotracheal intubation: a systematic review. Chest 2010;137:665-73. [Crossref] [PubMed]
- Tolep K, Getch CL, Criner GJ. Swallowing dysfunction inpatients receiving prolonged mechanical ventilation. Chest 1996;109:167-72. [Crossref] [PubMed]
- Chun BJ, Bae JS, Lee SH, et al. A prospective randomized controlled trial of the laryngeal mask airway versus the endotracheal intubation in the thyroid surgery: evaluation of postoperative voice, and laryngopharyngeal symptom. World J Surg 2015;39:1713-20. [Crossref] [PubMed]
- Kagotani R, Yoshida M, Muraki S, et al. Prevalence of diffuse idiopathic skeletal hyperostosis (DISH) of the whole spine and its association with lumbar spondylosis and knee osteoarthritis: the ROAD study. J Bone Miner Metab 2015;33:221-9. [Crossref] [PubMed]
- Kim BS, Moon MS, Yoon MG, et al. Prevalence of Diffuse Idiopathic Skeletal Hyperostosis Diagnosed by Whole Spine Computed Tomography: A Preliminary Study. Clin Orthop Surg 2018;10:41-6. [Crossref] [PubMed]
Cite this article as: Butler AJ, Ghasem A, Al Maaieh M. Dysphagia following lumbar spine surgery in the setting of undiagnosed DISH of the cervical spine: a case report. AME Case Rep 2019;3:13.


