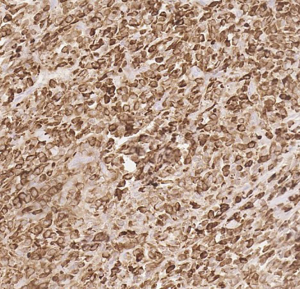
A rare case of TLE1-positive sclerosing epithelioid fibrosarcoma expanding the differential diagnosis of TLE1-positive tumors: a case report
Introduction
Transducin-like enhancer of split 1 (TLE1) is a transcription factor encoded by the TLE1 gene. Originally described in 1992 as human homologs of Drosophila Groucho proteins, TLE1 has been noted to play a role in many pathways including embryogenesis and inhibition of Wnt signaling (1,2). Its role in the Wnt pathway is especially relevant in synovial sarcoma where TLE1 was discovered to be upregulated. Early studies showed strong nuclear positivity on immunohistochemistry with high sensitivity and specificity for synovial sarcoma (2). Later studies revealed weak positivity in non-synovial sarcomas (3). These studies have most commonly shown TLE1 positivity in peripheral nerve sheath tumors and solitary fibrous tumors (2). However, occasional staining for TLE1 has also been described in clear cell sarcoma, high-grade chondrosarcoma, Ewing sarcoma, rhabdomyosarcoma, GIST, myxofibrosarcoma, and leiomyosarcoma (2). Staining in these tumors is typically focal and weak, to moderate in intensity, unlike the strong diffuse nuclear pattern characteristic of synovial sarcoma.
Case presentation
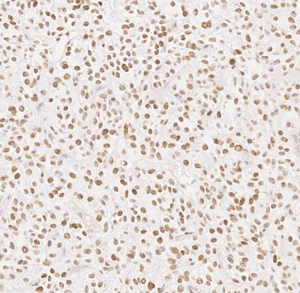
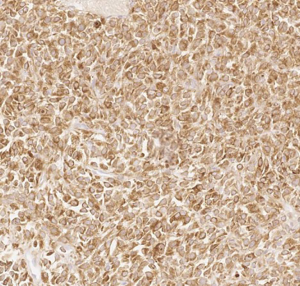
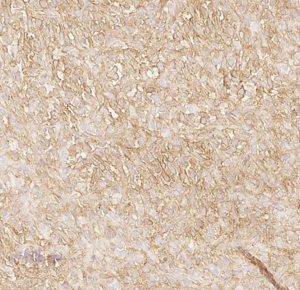
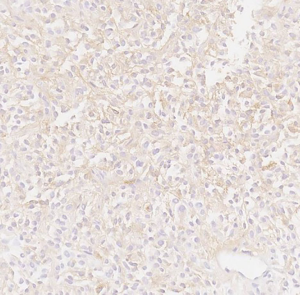
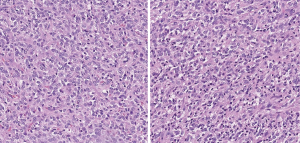
Here we present a case of TLE1 positivity in a case of sclerosing epithelioid fibrosarcoma (SEF). A 53-year-old male with a history of left femur bone sarcoma (diagnosed and resected in 2000 at an outside institution) with metastasis to the lung (diagnosed and resected in 2008 in an outside institution) presented with a new left posterior pelvic bone lytic lesion with soft tissue extension. Imaging also revealed suspicious hepatic and pancreatic masses. Biopsy of the soft tissue mass revealed a high-grade undifferentiated sarcoma with hypercellularity, densely packed epithelioid cells, and high vascularity (Figure 1). TLE1 (Figure 2), BCL2 (Figure 3), and CD99 (Figure 4) were uniformly positive, EMA was variably positive (Figure 5); S100, CK7, CK19, MyoD-1, myogenin, desmin, MART1, Stat 6, and CD34 were negative. SYT FISH study was attempted and was negative. Additional stains were performed in light of the negative SYT FISH studies: Cam5.2, DOG-1, chromogranin, synaptophysin, PAX-8, and CD117 were all negative. MUC4 was positive. The findings were overall most consistent with sclerosing epithelioid fibrosarcoma. Additionally, a battery of custom FISH testing done at an outside institution did not reveal abnormalities in CIC, BCOR-CCNB3, FUS and NCOA2. The latter assay was performed to exclude the possibility of a mesenchymal chondrosarcoma diagnosis.
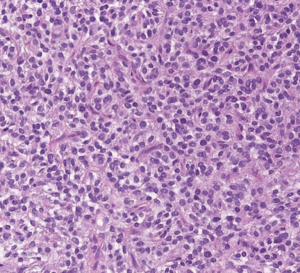
Consequently, the patient underwent a hemipelvectomy. The excision material was identical to the biopsy material, and MUC4 immunostain was repeated on the excision specimen and was diffusely positive (Figure 6); therefore, consistent with a sclerosing epithelioid fibrosarcoma (Figure 7).
Discussion
Sclerosing epithelioid fibrosarcoma (SEF) is a rare and malignant neoplasm categorized as a variant of fibrosarcoma. It is characterized by generally low-grade histologic features: small, cytologically bland nests and/or cords of epithelioid cells in a hyalinized stroma. First described in 1995, SEF typically presents in the fourth decade of life equally in both genders as a painful, slow growing mass of the deep soft tissues, often abutting the bones of the lower extremities or limb girdle (4,5). The immunohistochemistry profile includes positive MUC4 (sensitive and specific in up to 70% of cases), focal or weak positivity of EMA, S100, and cytokeratins, and negativity for CD34, leukocyte markers, HMB45, CD68, desmin, and SMA (5,6). The prognosis of SEF is generally poor, consistent with its malignant sarcomatous nature, whereby more than half of cases develop one or more local recurrences and more than 40% have metastases. Metastasis tends to occur in the lungs, pleura, and bone (5). At the molecular level, recent studies have found recurrent EWSR1-CREB3L1 fusion transcripts by reverse transcription polymerase chain reaction in 30% of pure SEF cases, splits and deletions of the EWSR1 and/or CREB3L1 genes by FISH in 60% of cases, and FUS-CREB3L2 gene fusion in 10% of hybrid SEF/LFFMS cases (7).
Monophasic synovial sarcoma was considered in the differential diagnosis of this case due to its strong TLE1 positivity. Synovial sarcoma (SS) is a mesenchymal spindle cell neoplasm which expresses epithelial differentiation. Monophasic SS is characterized by a spindle cell component or an epithelioid glandular or nested component. The immunophenotype of SS includes positivity for cytokeratins, EMA, CD99, Bcl-2, and occasionally S100 (8). Its specific and sensitive expression of TLE1 has been well documented. The majority of synovial sarcomas are characterized by the chromosomal translocation t(X;18). Ultimately in our case, the FISH did not detect the presence of any SYT gene abnormalities, thus arguing against a synovial sarcoma.
Epithelioid tumors, such as carcinomas, epithelioid sarcoma, epithelioid leiomyosarcoma, epithelioid angiosarcoma, and myoepithelial carcinoma were considered as a result of their morphology and EMA positivity. Acinar cell carcinoma was part of the differential for the mass found on imaging in the pancreas, with the pelvic mass a possible metastasis. These are rare malignant tumors of the pancreas that are usually solid but can also appear cystic. Histologically, there is clear evidence of acinar cell differentiation, which can be identified immunohistochemically by staining for trypsin, chymotrypsin, elastase, or lipase (9). Our specimen was negative for trypsin and chymotrypsin and ultimately, the pancreatic mass was biopsied and diagnosed as benign.
Epithelioid sarcomas (ES) are rare sarcomas of unknown lineage that are histologically classified as two subtypes: the conventional “distal” form, which can be mistaken for benign reactive processes due to epithelioid cells forming granuloma-like areas of necrosis and hyalinization in acral sites and the proximal “large cell” type, which consists of sheets and nests of large epithelioid cells in proximal sites. Immunohistochemically, ES is positive for cytokeratins, EMA, and CD34 (10). Our specimen was cytokeratin and CD34 negative, making this diagnosis unlikely. Angiosarcomas are malignant vascular tumors that can arise in soft tissues. Some of these tumors are classified as epithelioid angiosarcomas due to their predominant epithelioid histology and can mimic metastatic carcinomas. Besides expressing the usual vascular markers (CD34, CD31, ERG, FLI1) epithelioid angiosarcomas will also express cytokeratins and EMA (11). Epithelioid leiomyosarcomas are a rare histologic subtype of leiomyosarcoma that can arise in bone and often metastasize to the lungs. They invade in epithelioid sheets and express SMA and CD99 (12). Myoepithelial carcinomas in soft tissue are morphologically and immunophenotypically similar to the salivary gland counterparts. They are rare but affect all ages, most commonly on the limbs and limb girdles, as a painless superficial mass. These neoplasms stain positively for cytokeratins, S100, EMA, and calponin. SMA and p63 are occasionally positive (13). Our case was S100, cytokeratin, and cam5.2 negative, ruling out this diagnosis.
Other considerations included melanoma and malignant peripheral nerve sheath tumors. Metastatic melanoma being a great mimicker was considered in the differential diagnosis; however the absent S100 and MART-1 immunopositivity go against it. Malignant peripheral nerve sheath tumor (MPNST) was considered due to its occasional TLE1 positivity and focal glandular differentiation with expression of keratin, EMA, CEA, or chromogranin. It most commonly stains for CD99, S100, and p53 (14). Although our case stained strongly with CD99, it was S100 negative, making this diagnosis less likely.
At this point in time, TLE1 positivity in our SEF remains uncertain. However, some studies have revealed that analysis of beta-catenin-containing nuclear complexes showed the presence of a variety of proteins that bind RNA and/or influence RNA metabolism, including FUS among others. FUS/TLS and similar factors may integrate Wnt-mediated RNA transcription with effects on RNA processing, to exert more fine-grained control of the expression of genes associated with tumorigenesis (15). Given that TLE1 has been associated with the Wnt signaling pathway, and SEF is linked to FUS gene rearrangement in a percentage of cases, we speculate that a possible common pathway may have played a role in the presence of TLE1 immunopositivity in our SEF case.
In conclusion we are presenting the first known case, up to our knowledge, of TLE1 positive sclerosing epithelioid fibrosarcoma. This expands the differential diagnosis of TLE1 positive tumors. TLE1 immunostain should be always interpreted with caution when facing a poorly differentiated bone or soft tissue sarcoma with round cell and/or spindle cell morphology.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Stifani S, Blaumueller CM, Redhead NJ, et al. Erratum: Human homologs of a Drosophila Enhancer of split gene product define a novel family of nuclear proteins. Nat Genet 1992;2:343. [Crossref] [PubMed]
- Terry J, Nielsen TO. Synovial Sarcoma: Role of TLE1 as a Diagnostic Immunohistochemical Marker. Methods Cancer Diagn Ther Progn 2009;6:393-403.
- Kosemehmetoglu K, Vrana JA, Folpe AL. TLE1 expression is not specific for synovial sarcoma: A whole section study of 163 soft tissue and bone neoplasms. Mod Pathol 2009;22:872-8. [Crossref] [PubMed]
- Meis-Kindblom JM, Kindblom L, Enzinger FM. Sclerosing Epithelioid Fibrosarcoma. A variant of fibrosarcoma simulating carcinoma. Am J Surg Pathol 1995;19:979-93. [Crossref] [PubMed]
- Fletcher CD. WHO classification of tumors of soft tissue and bone: Sclerosing epithelioid fibrosarcoma. Lyon: IARC Press 2013.
- Doyle LA, Wang W, Cin PD, et al. MUC4 Is a Sensitive and Extremely Useful Marker for Sclerosing Epithelioid Fibrosarcoma. Am J Surg Pathol 2012;36:1444-51. [Crossref] [PubMed]
- Arbajian E, Puls F, Magnusson L, et al. Recurrent EWSR1-CREB3L1 gene fusions in sclerosing epithelioid fibrosarcoma. Am J Surg Pathol 2014;38:801-8. [Crossref] [PubMed]
- Fletcher CD. WHO classification of tumors of soft tissue and bone: Synovial sarcoma. Lyon: IARC Press 2013.
- Wisnoski NC, Townsend CM Jr, Nealon WH, et al. 672 Patients with Acinar Cell Carcinoma of the Pancreas: A Population-Based Comparison to Pancreatic Adenocarcinoma. Surgery 2008;144:141-8. [Crossref] [PubMed]
- Fletcher CD. WHO classification of tumors of soft tissue and bone: Epithelioid sarcoma. Lyon: IARC Press 2013.
- Deshpande V, Rosenberg AE, Oconnell JX, et al. Epithelioid Angiosarcoma of the Bone. Am J Surg Pathol 2003;27:709-16. [Crossref] [PubMed]
- Yamamoto T, Minami R, Ohbayashi C, et al. Epithelioid leiomyosarcoma of the external deep soft tissue. Arch Pathol Lab Med 2002;126:468-70. [PubMed]
- Fletcher CD. WHO classification of tumors of soft tissue and bone: Myoepithelial carcinoma. Lyon: IARC Press 2013.
- Rodriguez FJ, Folpe AL, Giannini C, et al. Pathology of peripheral nerve sheath tumors: diagnostic overview and update on selected diagnostic problems. Acta Neuropathol 2012;123:295. [Crossref] [PubMed]
- Bordonaro M. Crosstalk between Wnt Signaling and RNA Processing in Colorectal Cancer. J Cancer 2013;4:96-103. [Crossref] [PubMed]
Cite this article as: Perez D, Fullmer JM, Naous R. A rare case of TLE1-positive sclerosing epithelioid fibrosarcoma expanding the differential diagnosis of TLE1-positive tumors: a case report. AME Case Rep 2019;3:6.