Hodgkin’s lymphoma in a patient on adalimumab treatment for psoriasis
Introduction
Psoriasis is a chronic immune-mediated inflammatory skin disorder with genetic and environmental risk factors (1). Psoriasis varies according to age and geographic region, but the overall prevalence is about 2–4% in the adult population (2,3). Patients with psoriasis have a higher risk of malignancies, compared to those without the condition (4).
Pathogenesis in the development and maintenance of psoriasis-associated skin changes involves overproduction of tumor necrosis factor (TNF)-α, and interleukin (ILs)-23 and -17 (5). Psoriasis treatment has been revolutionized over the last decade with biologically targeted therapy for moderate to severe psoriasis, and several biological therapies have also been approved by the Food and Drug Administration (FDA), including TNF-alpha inhibitors; infliximab, etanercept, adalimumab, and certolizumab pegol, anti-IL-12/23; ustekinumab, anti-IL-17A; ixekizumab, secukinumab and brodalumab and IL-23 inhibitors; guselkumab and tildrakizuma. Biological therapies are generally well tolerated and have statistically significant efficacy and safety profiles (6,7). The risk of developing lymphoproliferative disease and other malignancies following treatment with anti-TNF agents, including adalimumab, is still a matter of debate: recent literature and case reports in patient with rheumatoid arthritis show an increased incidence of secondary malignancies during and after TNF-α inhibitor therapy (8,9); others show no evidence of an increased risk of cancer, except for non-melanoma skin cancer (NMSC) (10). In this report, we present a case of a young woman who suffered from chronic plaque psoriasis and who developed Hodgkin’s lymphoma five weeks after initiation of adalimumab.
Case presentation
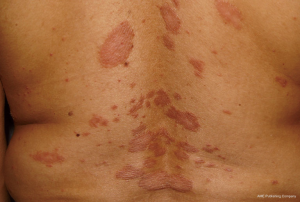
A 20-year-old Saudi female, with no other known medical conditions, presented to the Outpatient Dermatology Clinic of King Khalid University Hospital, Riyadh, on July 11th, 2017. This patient had a 12-year history of clinically diagnosed chronic plaque psoriasis, and inadequate response to topical therapies (Figure 1). Her medical history showed lower back pain, she was a non-(ex-smoker), and had no family history of psoriasis. Physical examination revealed a body mass index (BMI) of 31, with a psoriasis body surface area involvement of approximately 20%, occurring over the trunk and limbs. Nails showed pitting, and there was no joint swelling or arthritis. Her laboratory investigations were normal [complete blood count (CBC)] with differentials; liver function; renal profiles; lipid profiles; hepatitis markers; human immunodeficiency virus; QuantiFERON, and tuberculin skin tests, so we started treatment with adalimumab. The first dose of 80 mg (Humira, AbbVie, Inc.) was administered on July 25th, followed by a second dose of 40 mg one week later. The patient was kept on a dose of 40 mg every other week, and was referred to rheumatology for assessment of joint involvement.
On August 3rd, 2017, she was seen at the emergency department (ED) due to severe back pain, and was diagnosed and managed for low back pain. She missed her dermatology appointment at the one-month follow up, and did not reschedule her appointment.
On September 21st, 2017, the patient had her first visit to the rheumatology clinic, as a function of progressive worsening of her lower back pain and swelling of her hand and feet joints, along with unintentional weight loss of 12 kg versus the previous two months. The assessment concluded a diagnosis of nonresponsive and worsening psoriatic arthritis, at which point it was decided to discontinue adalimumab, repeat laboratory parameters [including antinuclear antibodies for rheumatoid factor (ANA RF) and X-rays of the hands, feet, lumbosacral spine, and pelvis]. The patient was switched to certolizumab and methotrexate.
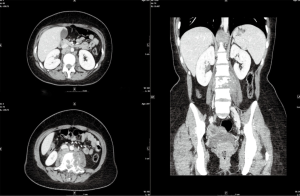
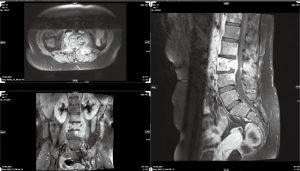
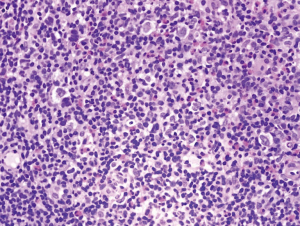
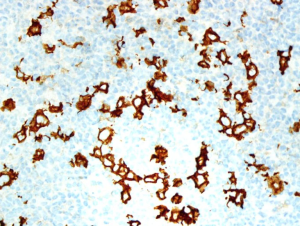
On October 4th, 2017, the patient returned to the rheumatology clinic with progressive and crippling back pain as well as severe buttock pain. She was using a wheelchair. The patient was admitted, and nuclear magnetic resonance imaging (NMRI) of the sacroiliac joint and spine was performed. The results were suggestive of lymphoma versus tuberculosis, whereas the high-resolution computed tomography (CT) was suggestive of lymphoma with bone involvement. This result was confirmed by CT-guided biopsy from the right posterior superior iliac spine, which confirmed bone marrow involvement, and was indicative of Hodgkin’s lymphoma (Figures 2-5). The patient was transferred to the care of the hematology-oncology service and started on the Adriamycin-Bleomycin-Vinblastine-Dacarbazine (ABVD) chemotherapy protocol, leading to complete clearance of psoriatic lesions after the first cycle.
Discussion
Psoriasis has been associated with a small increase in the risk of developing malignancies, including cutaneous lymphoma (11). This risk is connected to both the severity of the skin involvement and the duration of the psoriasis—factors which can lead to chronic lymphocyte stimulation, and eventually to the development of a dominant clone, which in turn promotes lymphoproliferative disorders with a higher relative risk of lymphoma (12,13).
Clinical psoriasis trials do not show an increased risk of malignancy (aside from NMSC) following treatment with TNF-α inhibitors (10,14,15). However, concern about TNF-α inhibitors and their association with cancer was triggered by post-marketing reports of 26 cases of lymphoma among patients treated with etanercept and infliximab (16). Another recent study, conducted by the large Psoriasis Longitudinal Assessment and Registry (PSOLAR) examined the safety of systemic treatments of psoriasis, finding a significant increase in the risk of malignancy, including lymphoma, following long-term (>12 months) treatment with a TNF-α inhibitor (17). It is not clear if this increase is due to the TNF-α inhibitor alone or attributable to a combination therapy. The FDA suggests that malignancy is a possible side effect of adalimumab (18), so it seems prudent to be extremely diligent for any signs of malignancy in this patient group.
Multiple studies show that cutaneous T-cell lymphoma (CTCL), including mycosis fungoides (MF), especially in the early stages, can resemble and be misdiagnosed as psoriasis, due to notable clinical and pathological overlaps between them (13,19). A recent case report showed a fast progression of CTCL to systemic T-cell lymphoma within weeks of starting adalimumab after an initial misdiagnosis of psoriasis (20). One study demonstrated the progression of cutaneous lymphoma after administration of anti-TNF-α therapy. In most cases, CTCL was present at the time of TNF-α inhibitor initiation (21). This highlights the importance of histological confirmation before biological therapy, as the latter will undermine the course of lymphoma and CTCL.
In the case presented here, the patient was first diagnosed clinically as having chronic plaque psoriasis and treated with topical therapy for 12 years, with no signs or symptoms of Hodgkin’s lymphoma. Given the short duration of five weeks from the point of initiation of treatment with the biologic agent, and the extremely aggressive behavior of lymphoma, we question the possibility of adalimumab as the cause of the Hodgkin’s lymphoma. One hypothesis in this case could be that the lesions were initially CTCL-associated skin lesions—which may mimic many diseases, including psoriasis. However, this would be unlikely, unless the patient had both psoriasis and Hodgkin’s lymphoma before starting adalimumab: as such, the behavior of the Hodgkin’s lymphoma became markedly more aggressive.
Given the case presented here, close monitoring of all patients receiving anti-TNF-α treatment, with a full report of any adverse outcomes, is highly recommended.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
References
- Capon F. The Genetic Basis of Psoriasis. Int J Mol Sci 2017;18:2526. [Crossref] [PubMed]
- Parisi R, Symmons DP, Griffiths CE, et al. Global epidemiology of psoriasis: A systematic review of incidence and prevalence. J Invest Dermatol 2013;133:377-85. [Crossref] [PubMed]
- Christophers E. Psoriasis – Epidemiology and clinical spectrum. Clin Exp Dermatol 2001;26:314-20. [Crossref] [PubMed]
- Reddy SP, Martires K, Wu JJ. The risk of melanoma and hematologic cancers in patients with psoriasis. J Am Acad Dermatol 2017;76:639-47.e2. [Crossref] [PubMed]
- Di Cesare A, Di Meglio P, Nestle FO. The il-23/th17 axis in the immunopathogenesis of psoriasis. J Invest Dermatol 2009;129:1339-50. [Crossref] [PubMed]
- Galván-Banqueri M, Gil RM, Ramos BS, et al. Biological treatments for moderate-to-severe psoriasis: Indirect comparison. J Clin Pharm Ther 2013;38:121-30. [Crossref] [PubMed]
- Jabbar-Lopez ZK, Yiu ZZN, Ward V, et al. Quantitative Evaluation of Biologic Therapy Options for Psoriasis: A Systematic Review and Network Meta-Analysis. J Invest Dermatol 2017;137:1646-54. [Crossref] [PubMed]
- Liu L, Charabaty A, Ozdemirli M. EBV-associated plasmablastic lymphoma in a patient with Crohn's disease after Adalimumab treatment. J Crohns Colitis 2013;7:e118-9. [Crossref] [PubMed]
- Wong AK, Kerkoutian S, Said J, et al. Risk of lymphoma in patients receiving antitumor necrosis factor therapy: A meta-analysis of published randomized controlled studies. Clin Rheumatol 2012;31:631-6. [Crossref] [PubMed]
- Peleva E, Exton LS, Kelley K, et al. Risk of Cancer in Patients with Psoriasis on Biologic Therapies: A Systematic Review. Br J Dermatol 2018;178:103-13. [Crossref] [PubMed]
- Gelfand JM, Shin DB, Neimann AL, et al. The risk of lymphoma in patients with psoriasis. J Invest Dermatol 2006;126:2194-201. [Crossref] [PubMed]
- Nograles KE, Davidovici B, Krueger JG. New insights in the immunologic basis of psoriasis. Semin Cutan Med Surg 2010;29:3-9. [Crossref] [PubMed]
- Nikolaou V, Marinos L, Moustou E, et al. Psoriasis in patients with mycosis fungoides: A clinicopathological study of 25 patients. J Eur Acad Dermatol Venereol 2017;31:1848-52. [Crossref] [PubMed]
- Papp KA, Poulin Y, Bissonnette R, et al. Assessment of the long-term safety and effectiveness of etanercept for the treatment of psoriasis in an adult population. J Am Acad Dermatol 2012;66:e33-45. [Crossref] [PubMed]
- Gordon K, Papp K, Poulin Y, et al. Long-term efficacy and safety of Adalimumab in patients with moderate to severe psoriasis treated continuously over 3 years: Results from an open-label extension study for patients from REVEAL. J Am Acad Dermatol 2012;66:241-51. [Crossref] [PubMed]
- Brown SL, Greene MH, Gershon SK, et al. Tumor necrosis factor antagonist therapy and lymphoma development: Twenty-six cases reported to the Food and Drug Administration. Arthritis Rheum 2002;46:3151-58. [Crossref] [PubMed]
- Fiorentino D, Ho V, Lebwohl MG, et al. Risk of malignancy with systemic psoriasis treatment in the Psoriasis Longitudinal Assessment Registry. J Am Acad Dermatol 2017;77:845-54.e5. [Crossref] [PubMed]
- Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/125057s0276lbl.pdf
- Reddy K, Bhawan J. Histologic mimickers of mycosis fungoides: A review. J Cutan Pathol 2007;34:519-25. [Crossref] [PubMed]
- Rohl R, Bax D, Schierer S, et al. A case for histologic verification of the diagnosis of atypical psoriasis before systemic therapy. JAAD Case Reports 2018;4:465-7. [Crossref] [PubMed]
- Martinez-Escala ME, Posligua AL, Wickless H, et al. Progression of undiagnosed cutaneous lymphoma after anti-tumor necrosis factor-alpha therapy. J Am Acad Dermatol 2018;78:1068-76. [Crossref] [PubMed]
Cite this article as: Alballa N, Alyousef A, Alamari A, Alhumidi AA, Zayed MA, Zeitouni L, Alsaif FM. Hodgkin’s lymphoma in a patient on adalimumab treatment for psoriasis. AME Case Rep 2018;2:49.