Sacral intraosseous lipoma: a case report
Introduction
Sacral tumors are rare and difficult to manage (1). The most common tumors include chordomas, giant cell tumors and metastatic lesions. Whilst surgery rarely benefits patients with metastatic disease, it is the principle treatment for patients with a range of rarer primary neoplasms. Furthermore, the nature and extent of surgical resection for lesions such as chordomas can have a determinative influence on patient outcome. Surgical excision of these lesions is challenging, and attendant morbidity high due to blood loss and damage to sacral nerve roots resulting in bowel and bladder dysfunction (2). Complex reconstructive techniques may be required. Finding the balance between a wide surgical resection to minimize local recurrence versus preserving pelvic stability and neurological function can present a difficult neurosurgical dilemma. A histological diagnosis using image guided needle biopsy may assist in developing a clear surgical strategy, however a non-diagnostic result can occur in up to 12% of patients, necessitating an open biopsy.
Intraosseous lipomas are rare benign bone tumors which originate from mature adipocytes. They are predominantly found in long bones and are seldom reported in the spine (3-5). Their diagnosis can be difficult due to the evolution of changes within the lesion which can produce confounding radiological and histological appearances (5-7).
The authors present a case report which illustrates the neurosurgical management of a patient with a complex sacral lipoma, to discuss the challenges in initial diagnosis and increase awareness about this rare clinical entity.
Case presentation
History
A 44-year-old female office worker presented with a history of progressive sacral pain radiating to her buttocks and posterior thighs. She reported worsening symptoms when sitting down for prolonged periods of time and when moving from sitting to standing. She had found physiotherapy ineffective and her pain was uncontrolled despite increasing analgesia. She denied any weakness or sensory deficits. In the last 12 months she had experienced frequency of micturition but no urinary or fecal incontinence.
On examination, she had no sacral tenderness or deformity. Her lower limb neurological examination was normal.
Investigations
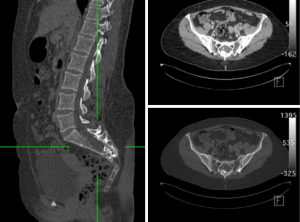
A CT lumbar spine was organized and revealed a 2 cm × 2.5 cm calcified destructive lesion arising from the posterior aspect of the sacrum and expanding the sacral canal. It involved the S3 posterior elements and had spiculated elements extending to S4 and S5 (see Figure 1).
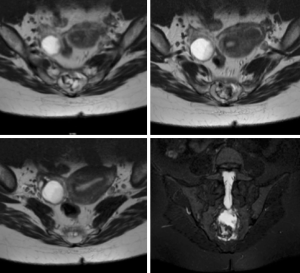
An MRI scan was conducted and demonstrated a heterogeneously enhancing lesion within the sacral canal which scalloped the S2 and S3 vertebral bodies. The lesion was predominantly hyper intense on T1, with areas T2 hyper intensity and areas of calcification (see Figure 2). There was no pre-sacral or para-sacral soft tissue component. The posterior T1–T2 hyper intense area which was suppressed on T1 fat saturated image anterior to the vertebral bodies was suggestive of a possible lipomatous component (see Figure 3).

The differential diagnoses were that of a sacral bony lesion including chordoma, chondrosarcoma, giant cell lesion or metastasis. The patient underwent two separate CT guided core biopsies which were inconclusive; showing remodeled bone and calcified marrow.
After extensive discussion a decision was made to perform an open biopsy, proceeding to a marginal resection at the time or a wide resection at a subsequent interval.
Operative findings
The tumor was resected using a posterior approach. Using intra-operative navigation based on an O-arm, multiple open biopsies were initially sent but the diagnosis could not be established on frozen section. It did not have features suggestive of a chordoma. A decision was made to proceed with marginal resection. Formal bilateral subperiosteal dissection of the paraspinal muscles was made to expose the S2 to S5 lamina. A piecemeal resection was performed including bony resection of a small margin of the normal sacrum beyond the visible boundary of the tumor. The tumor remained entirely extradural albeit adherent to that layer. Total macroscopic excision was achieved and confirmed visually and stereotactically. The structural integrity of the sacrum was sufficiently preserved. A small dural defect at S2 was repaired prior to closure.
Pathological findings
The histology revealed sections of remodeled bone and a small amount of surrounding soft tissue. Within the segments of bone, were large zones of fat necrosis, some of which showed lipomembranous change and extensive dystrophic calcification. This was associated with organizing granulation tissue. Fragments of paucicellular fibrous tissue were consistent with a pseudo capsule or portion of cyst wall. This was in a background of remodeled bone with scattered necrotic bony spicules. In places, the bone marrow stroma showed reactive fibromyxoid change with no significant inflammatory infiltrate. There was no evidence of malignancy. The histological features were predominantly of reactive changes with extensive dystrophic calcification. The appearances were consistent with a longstanding process with superimposed reactive changes (see Figure 4).

Post-operative course
After correlating the histology results with the MRI findings, a diagnosis of a necrotic intraosseous lipoma was made. Post operatively the patient made a good symptomatic recovery and is being managed expectantly.
Discussion
An intraosseous lipoma is an entity which was first reported in 1910 by Wehrsig and later classified according to its different stages of evolution by Milgram (see Table 1) (3,6). The variability in its appearance can cause diagnostic difficulty and the differential diagnoses include both benign and malignant bony tumors, cysts and infarction. The incidence is thought to be less than 0.1% of all primary bone tumors and its etiology is thought to be associated with possible history of trauma or bony infarct. Whilst the entity is most common in the calcaneum and long limb bones, there are few reports of intraosseous lipoma within the axial skeleton (4,6,8,9). At most locations, they are asymptomatic or benign in nature but in areas of the spine they can cause pain or neurological deficits due to the expansile nature of the lesion, requiring awareness amongst spinal surgeons (3).
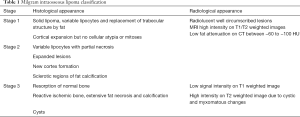
Full table
The pathology of the disease is very much that of an evolving lesion which results in bony expansion as normal bone marrow is eventually replaced by fat. The histological features can be non-specific and include varying areas of fat necrosis, inflammation and calcification or sclerosis due to gradual compression of the expanding lipoma within the bony cavity (6,10). Macroscopically, they can resemble normal fat in Milgram’s early stages I and II but undergo necrosis and develop cysts and calcification at stage III as a result of myxomatous degeneration and infarction within the lipoma. As illustrated in our case, an initial biopsy was thought to be inconclusive based on the areas of dystrophic calcification and inflammation and necrosis which in retrospect was likely a stage III necrotic intraosseous lipoma.
The correlation between the histological and radiological findings was essential in confirming diagnosis in our case. The MRI findings on intraosseous lipomas have been reported in long bones to be predominantly similar to MRI findings for adipose tissue. Typically, in stages I and II of the lesion, where fat is the main component, we can expect homogenous hyper intensity on T1 and T2 weighted images. However, as the lesion progresses to stage III and develops areas of necrosis and ischemia, the findings can be more heterogenous with low signal intensity on T1 weighted images and high signal intensity on T2 weighted images due to cystic and myxomatous changes. Having a short-T inversion recovery (STIR) sequence can be useful to differentiate a haematoma which also has high signal intensity on STIR (3,7).
This case report illustrates the management of a patient with a sacral lipoma which required surgical intervention for diagnostic purposes and symptom control. The diagnosis of a sacral lipoma could be made based on histological and radiological appearances but proved challenging. Clinicians fared with a patient with a destructive sacral lesion should be aware of that entity, particularly when considering inherently morbid surgical strategies relevant for other pathologies.
Acknowledgements
The authors wish to acknowledge Dr. Alison Cheah for her contribution in reviewing and providing the pathology slides.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Informed voluntary consent has been obtained from the patient and clinicians involved for publication of the manuscript.
References
- Whittaker LD, Pemberton JD. Tumors ventral to the sacrum. Ann Surg 1938;107:96-106. [Crossref] [PubMed]
- Zhang ZY, Fu CF, Yang YX, et al. Long-term outcomes following en bloc resection for sacral tumor: a retrospective analysis of 93 cases. Orthopedics 2011;34:e403-7. [PubMed]
- Milgram JW. Intraosseous lipomas: radiologic and pathologic manifestations. Radiology 1988;167:155-60. [Crossref] [PubMed]
- Bridge JA, Rosenberg AE. Lipoma of bone. In: Fletcher CD, Unni KK, Mertens F, et al. editors. WHO classification of tumours: pathology and genetics of tumours of soft tissue and bone: Lyon: IARC Press, 2002:328-9.
- Eyzaguirre E, Liqiang W, Karla GM, et al. Intraosseous lipoma. A clinical, radiologic, and pathologic study of 5 cases. Ann Diagn Pathol 2007;11:320-5. [Crossref] [PubMed]
- Milgram JW. Intraosseous lipomas. A clinicopathologic study of 66 cases. Clin Orthop Relat Res 1988.277-302. [PubMed]
- Blacksin MF, Ende N, Benevenia J. Magnetic resonance imaging of intraosseous lipomas: a radiologic-pathologic correlation. Skeletal Radiol 1995;24:37-41. [Crossref] [PubMed]
- Hanelin LG, Sclamberg EL, Bardsley JL. Intraosseous lipoma of the coccyx. Report of a case. Radiology 1975;114:343-4. [Crossref] [PubMed]
- Ehara S, Kattapuram SV, Rosenberg AE. Case report 619. Intraosseous lipoma of the sacrum. Skeletal Radiol 1990;19:375-6. [Crossref] [PubMed]
- Chow LT, Lee KC. Intraosseous lipoma. A clinicopathologic study of nine cases. Am J Surg Pathol 1992;16:401-10. [Crossref] [PubMed]
Cite this article as: Li Ching Ng A, Davies M. Sacral intraosseous lipoma: a case report. AME Case Rep 2018;2:33.


