A rare case of symptomatic hyperparathyroidism in an elderly patient with dual pathology
Introduction
Parathyroid cancer (PTCa) is extremely rare. It is responsible for only 0.005% of all cancers (1) and has an incidence rate of 0.15 per 1,000,000 individuals per year (2). The median age of occurrence is 56 years and it occurs equally between males and females (3,4). It is responsible for <1% of cases of primary hyperparathyroidism (3,5). The most common cause of primary hyperparathyroidism is parathyroid adenoma (PTa) (93.0%). Unlike PTCa, it is more common in women and the elderly (6). Few cases of co-existent PTCa and PTa have been reported, and usually in the context of an underlying familial disease process (7,8). Here, we present a case of a true sporadic synchronous PTCa and PTa in an elderly patient.
Case presentation
An 80-year-old woman presented to hospital with a 6-month history of constipation and abdominal pain. She was recently diagnosed with hypercalcaemia and referred to an endocrinologist after routine blood work from her general practitioner revealed a corrected serum calcium (Ca) of 3.39 mmol/L (normal range, 2.10–2.55 mmol/L), parathyroid hormone (PTH) of 52 pmol/L (normal range, 1.2–9.3 pmol/L), serum phosphate (PO4) of 0.64 mmol/L (normal range, 0.74–1.50 mmol/L) and alkaline phosphatase (ALP) of 94 IU/L (normal range, 40–150 IU/L). Her only co-morbidity of note was hypertension for which she received angiotensin-converting enzyme (ACE) inhibitor therapy. There was no known family history of endocrine disease. During her hospital stay physical examination did not reveal any systemic abnormality, no neck masses and no lymphadenopathy.
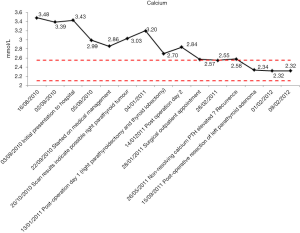
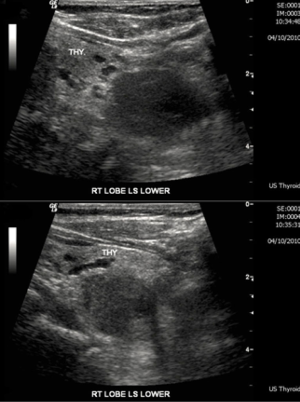
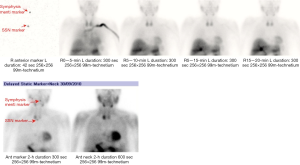
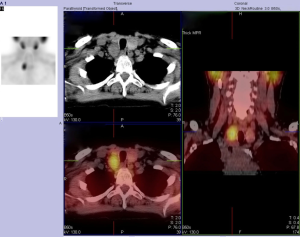
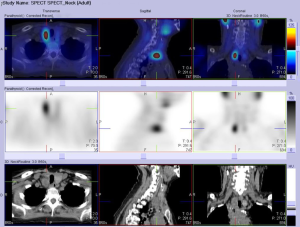
On admission, she was treated with fluid rehydration, diuretics and bisphosphonates. Investigations revealed a decrease in Ca to 2.86 mmol/L, serum PTH of 44 pmol/L, a negative myeloma screen and normal levels of tumour markers cancer antigen 125 (CA125) and carcinoembryonic antigen (CEA). Remaining bloods showed no abnormality. She was referred for localisation imaging for suspected parathyroid adenoma and underwent an ultrasound of the neck, sesta-MIBI scan and single-photon emission computed tomography-computed tomography (SPECT-CT). Neck ultrasound revealed a well-demarcated, solid, hypoechoic, 26-mm mass posterior to the lower third of the right lateral lobe (Figure 1). A sesta-MIBI highlighted conspicuous activity over the lower right neck on the 2-hour images (Figure 2). SPECT scan (Figures 3,4) showed an enlarged right inferior parathyroid gland with increased radioisotope uptake. All three findings were in keeping with a right inferior PTa. Hence, the working diagnosis was primary hyperparathyroidism secondary to a right-sided inferior PTa. The patient was referred to the surgeons and arrangements were made for a minimally invasive right sided parathyroidectomy.
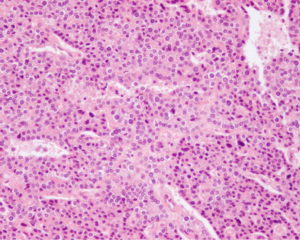
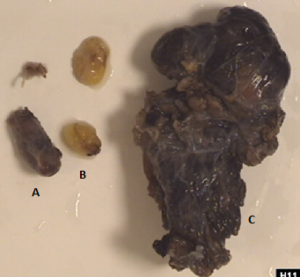
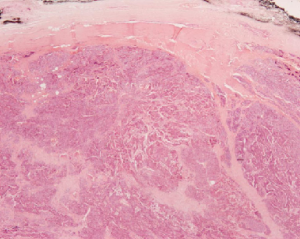
During surgery the gland was located but was very difficult to dissect and showed signs of infiltration into the thyroid gland. Visualisation of the anatomy became difficult and given the suspicion of malignancy it was decided to convert to a conventional open approach via a midline scar. The patient underwent an en-bloc resection of the right inferior parathyroid and right thyroid lobe (Figure 5). Intra-operative examination of the surrounding tissue, including the laryngeal nerve and strap muscles, showed no signs of infiltration. Post-operative recovery was unremarkable and the patient was discharged home on day 2 with a Ca of 2.7 mmol/L and a PTH of 10 pmol/L. Histopathological findings were in keeping with intra-operative findings and a diagnosis of PTCa of the right inferior gland was made (Figures 6,7). Diagnosis of PTCa is always difficult histologically (9,10). In our specimen, the mitotic count was considered to be low for a carcinoma, however the dense fibrous bands and infiltrative architecture favoured the diagnosis. Immunostaining for CD34 and factor VIII confirmed endothelial lined space invasion. The Ki67 proliferation index staining is approximately 5%.
The patient was reviewed in clinic 2 weeks later. Blood tests revealed some resolution of the hypercalcaemia (2.57 mmol/L) and normal thyroid function. She was reassured that the margins of the resection were clear and underwent a plain chest radiograph and MRI of the neck, which were both reported as normal. Radiotherapy was discussed at the multidisciplinary team meeting (MDT) and concluded not to be of clinical benefit in this situation.
She was placed on close observation with serial Ca and PTH level monitoring over the next few months (Figures 8,9). The case was reviewed again in MDT 6 weeks post-operatively. At this point the PTH had risen to 11.2 pmol/L from 10.2 pmol/L with a Ca of 2.55 mmol/L since the previous month. Concern was raised as to the source of the raised parathyroid activity given the previous carcinoma and a repeat sesta-MIBI scan was performed. This revealed no uptake in the bed of the right thyroid; however, activity was noted in the left lateral lobe of the thyroid and the left half of the isthmus. SPECT-CT revealed a small 11-mm left paraoesophageal mass behind the lower left lateral thyroid lobe suggestive of a left inferior parathyroid adenoma. She was counseled as to the result and advised to undergo a repeat operation to remove the parathyroid tumour.
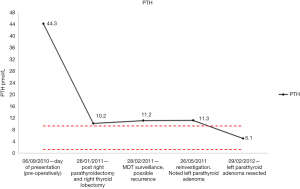
The operation was via an open approach and the left inferior parathyroid gland was removed without complication as a day case procedure. Histopathologically, a hypercellular parathyroid gland composed of a mixture of chief cells, water clear cells and oxyphilic cells arranged as microfollicles, sheets and nodules were seen. These features are consistent with a PTa. No features of malignancy were observed. Post-operative review showed good function of her two remaining parathyroid glands with a Ca level of 2.30 mmol/L, PTH level of 5.1 pmol/L and thyroid stimulating hormone (TSH) level of 1.49 mU/L (normal range, 0.35–5.00 mU/L). Given the rare nature of her synchronous parathyroid tumours she was further investigated to check whether these were sporadic or part of a syndrome. Two years on from her diagnosis she is well and remains disease free with calcium and PTH within the normal range.
Discussion
Functional PTCa occurrence is well documented, however, its sporadic synchronous occurrence in the presence of PTa in individuals with no identifiable risk factors has rarely been published. To our knowledge, only three (7,11,12) clearly documented cases have been previously reported (Table 1), with one case of non-functioning PTCa and PTa (13).

Full table
Diagnosing malignancy is difficult pre- and post-operatively. Patients with malignancy are more likely to be symptomatic of hypercalcaemia, present with Ca levels of >3.5 mmol/L and PTH levels of >3–10 times the upper limit of normal or a palpable neck mass, lymphadenopathy or recurrent laryngeal nerve palsy (3). However, individuals with benign disease may also present similarly (3). Our patient did present with symptoms of hypercalcaemia however, due to a Ca of 3.39 mmol/L, absence of a neck lump or lymphadenopathy and rarity of PTCa as the aetiology of primary hyperparathyroidism, it was presumed that the primary hypercalcaemia in our patient was secondary to a PTa.
The first suspicion of malignancy versus adenoma became apparent intraoperatively when infiltration of the tumour into the thyroid gland was visualised. The tumour was dark greyish-brown and firm (Figure 5). These are features consistent with carcinoma whereas adenomas are non-invasive, a tan-dark yellow colour and soft (14). Histologically, the collective presence of a fibrous trabecular architecture, vascular and capsular invasion, mitotic activity or aneuploidy suggests carcinoma (9,15-17). In our patient, the presence of a fibrous trabecular architecture with vascular and capsular invasion (Figures 6,7) confirmed PTCa diagnosis.
Suspecting and treating PTCa early is essential. This is because complete resection of the carcinoma independently affects disease-free survival (15,17). Unfortunately, difficulties in diagnosing malignancy results in patients presenting years after initial resection with symptoms of hypercalcaemia and metastasis (2,9,10). At this stage mortality is high (15). Hence, although PTCa is an uncommon cause of primary hyperparathyroidism, it is important to suspect and exclude it. Routine bilateral neck exploration with identification of all four parathyroid glands should be performed in individuals with suspected PTCa to minimise this risk (12).
Cancer occurrence in conjunction with adenomatous disease is more commonly reported in the context of underlying familial hyperparathyroidism (18,19), i.e., familial isolated hyperparathyroidism, jaw tumour syndrome, multiple endocrine neoplasia type 1 (MEN1) and multiple endocrine neoplasia type 2A (MEN2A) syndromes (20-22). We retrospectively analysed head and neck images of our patient to look for evidence of ossifying jaw or pituitary tumours; serum calcitonin levels were measured (and normal) and the history for uncontrolled hypertension or family history of hyperparathyroidism were clarified. None of these were evident suggesting clinically non-syndromic primary hyperparathyroidism. Given its rarity, genetic testing was not performed. Prior neck radiation and chronic renal failure have also been associated with synchronous PTCa and PTa (3,23,24). Our patient had no history of these conditions, leading us to recognize the case as a true sporadic case of synchronous parathyroid tumours.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Hundahl SA, Fleming ID, Fremgen AM, et al. Two hundred eighty-six cases of parathyroid carcinoma treated in the U.S. between 1985-1995: a National Cancer Data Base Report. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer 1999;86:538-44. [Crossref] [PubMed]
- Fraker DL. Parathyroid Tumors. In: DeVita VT Jr, Hellman S, Rosenberg SA. editors. Cancer: Principles and Practice of Oncology. 7th edition. Philadelphia, Pa: Lippincott Williams & Wilkins, 2005:1521-27.
- Shane E. Clinical review 122: Parathyroid carcinoma. J Clin Endocrinol Metab 2001;86:485-93. [Crossref] [PubMed]
- Lee PK, Jarosek SL, Virnig BA, et al. Trends in the incidence and treatment of parathyroid cancer in the United States. Cancer 2007;109:1736-41. [Crossref] [PubMed]
- Koea JB, Shaw JH. Parathyroid cancer: biology and management. Surg Oncol 1999;8:155-65. [Crossref] [PubMed]
- Fuleihan GE, Arnold A. Parathyroid carcinoma. Available online: http://www.uptodate.com/contents/parathyroid-carcinoma?source=search_result&search=parathyroid+cancer&selectedTitle=1%7E16
- Shapiro DM, Recant W, Hemmati M, et al. Synchronous occurrence of parathyroid carcinoma and adenoma in an elderly woman. Surgery 1989;106:929-33. [PubMed]
- Ito Y, Iwase H, Tanaka H, et al. Metachronous primary hyperparathyroidism due to a parathyroid adenoma and a subsequent carcinoma: report of a case. Surg Today 2001;31:895-8. [Crossref] [PubMed]
- Smith JF, Coombs RR. Histological diagnosis of carcinoma of the parathyroid gland. J Clin Pathol 1984;37:1370-8. [Crossref] [PubMed]
- Bondenson L, Grimelius L, DeLellis RA, et al. Parathyroid carcinoma. In: DeLellis RA, Lloyd RV, Heitz PU, et al. editors. Pathology and Genetics of Tumours of Endocrine Organs. 3rd edition. WHO Classification of Tumours. Lyon, France: IARC Press, 2004:124-7.
- Goldfarb M, O'Neal P, Shih JL, et al. Synchronous parathyroid carcinoma, parathyroid adenoma, and papillary thyroid carcinoma in a patient with severe and long-standing hyperparathyroidism. Endocr Pract 2009;15:463-8. [Crossref] [PubMed]
- Chatterjee S, Ray U, Gupta S, et al. Concurrent parathyroid carcinoma and adenoma: A rare presentation of a rarer disease entity. Indian J Endocrinol Metab 2013;17:939-41. [Crossref] [PubMed]
- Ashkenazi D, Elmalah I, Rakover Y, et al. Concurrent nonfunctioning parathyroid carcinoma and parathyroid adenoma. Am J Otolaryngol 2006;27:204-6. [Crossref] [PubMed]
- Clark O. Parathyroid carcinoma. In: Doherty GM, Way LW. editors. Current surgical diagnosis and treatment. Michigan: McGraw-Hill Companies, 2006:284-93.
- Wei CH, Harari A. Parathyroid carcinoma: update and guidelines for management. Curr Treat Options Oncol 2012;13:11-23. [Crossref] [PubMed]
- Schantz A, Castleman B. Parathyroid carcinoma. A study of 70 cases. Cancer 1973;31:600-5. [Crossref] [PubMed]
- Sandelin K, Auer G, Bondeson L, et al. Prognostic factors in parathyroid cancer: a review of 95 cases. World J Surg 1992;16:724-31. [Crossref] [PubMed]
- Dinnen JS, Greenwoood RH, Jones JH, et al. Parathyroid carcinoma in familial hyperparathyroidism. J Clin Pathol 1977;30:966-75. [Crossref] [PubMed]
- Mallette LE, Bilezikian JP, Ketcham AS, et al. Parathyroid carcinoma in familial hyperparathyroidism. Am J Med 1974;57:642-8. [Crossref] [PubMed]
- Marx SJ, Simonds WF, Agarwal SK, et al. Hyperparathyroidism in hereditary syndromes: special expressions and special managements. J Bone Miner Res 2002;17 Suppl 2:N37-43. [PubMed]
- Kebebew E. Parathyroid carcinoma, a rare but important disorder for endocrinologists, primary care physicians, and endocrine surgeons. Thyroid 2008;18:385-6. [Crossref] [PubMed]
- Sharretts JM, Simonds WF. Clinical and molecular genetics of parathyroid neoplasms. Best Pract Res Clin Endocrinol Metab 2010;24:491-502. [Crossref] [PubMed]
- Berland Y, Olmer M, Lebreuil G, et al. Parathyroid carcinoma, adenoma and hyperplasia in a case of chronic renal insufficiency on dialysis. Clin Nephrol 1982;18:154-8. [PubMed]
- Pai SI, Goldstein BJ, Studeman KD, et al. Concurrent sporadic parathyroid adenoma and carcinoma. Am J Otolaryngol 2006;27:346-8. [Crossref] [PubMed]
Cite this article as: Khan S, Sekhon H, Mihai R, Jenkins S. A rare case of symptomatic hyperparathyroidism in an elderly patient with dual pathology. AME Case Rep 2018;2:22.