Concurrent pulmonary benign metastasizing leiomyoma and primary lung adenocarcinoma: a case report
Introduction
Pulmonary benign metastasizing leiomyoma (BML) is a very rare metastatic phenomenon characterized by the growth of uterine leiomyoma tissue in the lung. There are only scattered case reports describing the imaging, pathogenetic mechanism, treatment and prognosis of the disease. Concurrent BML and primary lung cancer is even more uncommon. To the best of our knowledge, there are only two cases in the literature about the association between lung cancer and BML other than this report (1,2). Herein, we report a case of pulmonary BML associated with primary lung adenocarcinoma in a young woman and briefly review the literature.
Case presentation
A 38-year-old Chinese never-smoker female was admitted to our hospital on Jul 28, 2017, for incidental detection of lung opacity during physical examination 1 week ago. There was no special sign and symptom and the patient complained of a history of uterine leiomyoma resection 5 years ago. Laboratory test is normal. The hormone levels of patients include luteinizing hormone, follicle stimulating hormone, estradiol matched the corresponding year of the patient.
Chest computed tomography (CT) scan showed (Figure 1A) a ground-glass opacity (GGO) nodule coexisted with a solid mass (Figure 1B,C). We considered that the solid mass was a low grade malignant tumor, such as neuroendocrine tumor (carcinoid); and the GGO was suspected the early adenocarcinoma and we recommended follow-up after 6 months according with the Fleischner guideline in 2017 (3).
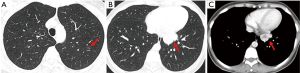
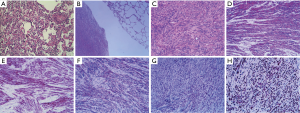
The patient underwent thoracoscopic wedge resection both the left upper lung and the left lower lung on Aug 10, 2017. The pathological diagnosis of the GGO was micro-invasive adenocarcinoma (MIA) and mild hyperplasia of peripheral alveolar epithelium showed epithelial structures composed of alveoli or bronchiole (Figure 2A). On the other hand, the pathological examination of the mass demonstrated spindle-shaped smooth muscle cells (Figure 2B,C) and the tumor cells were arranged in a cross fascicular arrangement, containing abundant eosinophilic cytoplasm, no cytological atypia, necrosis and mitotic figures. Strong positive immunohistochemical staining suggested for smooth muscle actin (SMA), h-caldesmon, desmin, estrogen receptor (ER) and progesterone receptor (PR) (Figure 2D,E,F,G,H). There was no expression of CD117, CD34, S-100, CD10, inhibin-α and CD99. It accords with the diagnostic criteria of benign metastasizing leiomyoma combined with a history of uterine leiomyoma.
Subsequently, the patient was discharged 5 days after surgery and had taken oral tamoxifen until Jan 1, 2018. Follow-up chest radiographs and CT showed no recurrence or metastasis at the writing.
Discussion
BML is a rare disease that usually occurs in women of reproductive age. In most cases, there is a previous history of myomectomy or hysterectomy for uterine leiomyoma. The tumor involves the lung most frequently. However, BML can metastasize to other distant organs such as the lymph nodes, skin, mediastinum, bone (especially spine), muscular tissue, retroperitoneum, central nervous system, heart.
The first case was reported by Steiner et al. (4) in 1939 in which a leiomyoma transferred from the uterus to the lung. Since then there have been multiple single or cases reports (5-7). Thus, we present the 3rd case of BML accompanied with primary lung adenocarcinoma.
Most patients with BML are usually asymptomatic and occasionally symptoms of chest pain, shortness of breath, and cough may be present. Pulmonary BML lesions tend to appear several years after the resection of the uterine leiomyomas. It was reported that the interval between hysterectomy and the diagnosis of BML ranged from 0 to 24 years (8). Miller et al. (9) reported the mean age at diagnosis BML was 54.1 years, while Barnaś et al. described the diagnosis was 47.3 years (7).
Imaging features of BML are often non-specific presenting bilateral multiple nodules/masses on chest radiography and CT. A miliary pattern or a pattern simulating interstitial lung disease have been reported occasionally (10). Cavitation and calcification are rarely showed. There is hardly mediastinal and hilar lymphadenopathy (11). For the differential diagnosis, both primary leiomyoma and metastases of highly differentiated leiomyosarcomas of the lung have to be excluded (12).
The pathogenesis of the tumor is unclarified. The several possible mechanisms include hormone-sensitive in situ proliferations of smooth muscle bundles, a benign smooth muscle cells from uterine leiomyoma transported and colonized in the lung, and metastasis from very low-grade uterine leiomyosarcoma (13,14). The patients demonstrated strong positivity for SMA, desmin, h-caldesmon, and ER/PR, confirming the diagnosis of uterine leiomyomas.
The diagnosis of this tumour depends on the histopathological examination. Lung biopsy is a simple and reliable method for diagnosis. It can be obtained by CT guided percutaneous lung biopsy, thoracoscopic biopsy, and thoracotomy lung biopsy. A standard treatment for BML has not yet been established. Management primarily consists of diagnostic resection with subsequent observation with or without hormonal suppression for residual pulmonary nodules (9) Because of the hormone-sensitive characteristics of BML, treatments are based on hormonal manipulation along with either surgical or medical oophorectomy (15). Our patient was successfully treated with curative excision and received adjuvant hormonal therapy. A ‘wait-and-see’ strategy is still needed to observe and follow up the patients with multiple metastasis of BML (15), in order to avoiding the deterioration and recurrence of the tumor.
In conclusion, we report a very rare case of BML associated with primary lung adenocarcinoma. When a solitary lung nodule/mass is detected in a patient with a history of myomectomy, pulmonary BML should be considered in the differential diagnosis. On the other hand, GGO is still the hot spot and needs to be identified carefully. Further studies are needed to improve the potential pathological mechanism, treatment and prognosis of BML.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Verbal informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Poujade O, Genin AS, Dhouha M, et al. A benign metastasizing leiomyoma involving a nodule in the pulmonary artery: case and literature review. Eur J Gynaecol Oncol 2010;31:329-32. [PubMed]
- Naito M, Kobayashi T, Yoshida M, et al. Solitary pulmonary nodule of benign metastasizing leiomyoma associated with primary lung cancer: a case report. J Med Case Rep 2011;5:500. [Crossref] [PubMed]
- MacMahon H, Naidich DP, Goo JM, et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology 2017;284:228-43. [Crossref] [PubMed]
- Steiner PE. Metastasizing fibroleiomyoma of the uterus: Report of a case and review of the literature. Am J Pathol 1939;15:89-110.7.
- Wei WT, Chen PC. Benign metastasizing leiomyoma of the lung: A case report and literature review. Oncol Lett 2015;10:307-12. [Crossref] [PubMed]
- Chen S, Zhang Y, Zhang J, et al. Pulmonary benign metastasizing leiomyoma from uterine leiomyoma. World J Surg Oncol 2013;11:163. [Crossref] [PubMed]
- Barnaś E, Książek M, Raś R, et al. Benign metastasizing leiomyoma: A review of current literature in respect to the time and type of previous gynecological surgery. PLoS One 2017;12:e0175875. [Crossref] [PubMed]
- Egberts JH, Schafmayer C, Bauerschlag DO, et al. Benign abdominal and pulmonary metastasizing leiomyoma of the uterus. Arch Gynecol Obstet 2006;274:319-22. [Crossref] [PubMed]
- Miller J, Shoni M, Siegert C, et al. Benign Metastasizing Leiomyomas to the Lungs: An Institutional Case Series and a Review of the Recent Literature. Ann Thorac Surg 2016;101:253-8. [Crossref] [PubMed]
- Chen S, Liu RM, Li T. Pulmonary benign metastasizing leiomyoma: a case report and literature review. J Thorac Dis 2014;6:E92-8. [PubMed]
- Abramson S, Gilkeson RC, Goldstein JD, et al. Benign metastasizing leiomyoma: clinical, imaging, and pathologic correlation. AJR Am J Roentgenol 2001;176:1409-13. [Crossref] [PubMed]
- Kayser K, Zink S, Schneider T, et al. Benign metastasizing leiomyoma of the uterus: documentation of clinical, immunohistochemical and lectin-histochemical data of ten cases. Virchows Arch 2000;437:284-92. [Crossref] [PubMed]
- Patton KT, Cheng L, Papavero V, et al. Benign metastasizing leiomyoma: clonality, telomere length and clinicopathologic analysis. Mod Pathol 2006;19:130-40. [Crossref] [PubMed]
- Lee HJ, Choi J, Kim KR. Pulmonary benign metastasizing leiomyoma associated with intravenous leiomyomatosis of the uterus: clinical behavior and genomic changes supporting a transportation theory. Int J Gynecol Pathol 2008;27:340-5. [Crossref] [PubMed]
- Ki EY, Hwang SJ, Lee KH, et al. Benign metastasizing leiomyoma of the lung. World J Surg Oncol 2013;11:279. [Crossref] [PubMed]
Cite this article as: Chen A, Sun T, Pu X, Li H, Yu T, Yu H. Concurrent pulmonary benign metastasizing leiomyoma and primary lung adenocarcinoma: a case report. AME Case Rep 2018;2:18.


