Open window thoracostomy as an alternative approach to secondarily infected malignant pleural effusion and failure of intrapleural catheter drainage: a case report
Introduction
Malignant pleural effusion (MPE) is a common manifestation of advanced lung cancer, with approximately 150,000 cases in the United States annually (1). MPE develops in approximately 30% of patients with advanced disease (2). Management of this clinical entity is largely palliative in nature, understanding that interventions to manage the effusion do not improve overall survival. However, currently we are in an expanding era of new treatment options in the management of advanced cancer that have not been seen before. With survival as a moving target, chronic problems become an increased concern. The ultimate goal of MPE treatment in this growing population of prolonged life expectancy is relief of dyspnea and prevention of fluid accumulation.
Treatment options for MPE include therapeutic thoracentesis, chemical pleurodesis (talc, bleomycin, tetracycline, etc.), indwelling pleural catheter (IPC), and pleuroperitoneal shunting (3). Choice of approach is a multifactorial decision with consideration of potential symptomatic benefit, rate of fluid accumulation, ability of the lung to expand to the chest wall, and predicted patient survival (4). IPC’s are becoming increasingly utilized to manage these effusions given their ease of placement as a same-day procedure, patient control of drainage based on symptoms at home, and spontaneous pleurodesis (SP) in 23–47% of patients (5,6). A recent randomized controlled trial illustrated that IPC’s are just as effective as chemical pleurodesis in controlling patient dyspnea, while also requiring fewer secondary pleural procedures (7).
Complications of IPC placement include catheter-associated pain, catheter dislodgment, development of fluid loculation, and infection (8). Incidence of secondarily infected pleural fluid after IPC placement has become an area of clinical interest, particularly because management of this rare clinical scenario is heterogeneous (9-12) and largely not driven by robust literature. Here we report a case of IPC placement for MPE with secondary infection, ultimately culminating in open window thoracostomy (OWT) for definitive drainage. We subsequently review the management of this rare clinical scenario, including our experience with open drainage.
Case presentation
The patient is a pleasant 74-year-old male with a history of non-small cell lung cancer who underwent video-assisted thoracoscopic (VATS) right upper lobe resection with mediastinal lymph node dissection, for stage IA non-small cell lung cancer (Figure 1). Nine months after definitive surgical resection, the patient developed progressive dyspnea and was found to have an MPE (Figure 2) on cytologic testing.

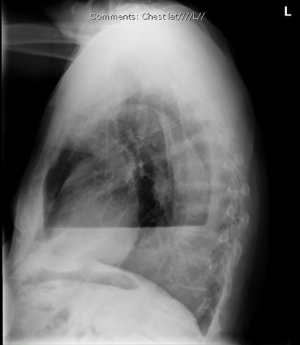
His MPE was initially managed with intermittent thoracentesis, however increased frequency of required drainage procedures led to the placement of a PleurX catheter (BD technologies; Franklin Lakes, NJ, USA). After repeated dislodgements of the IPC at home necessitating re-insertion, his pleural fluid became purulent. Lab analysis demonstrated pan-sensitive Pseudomonas aeruginosa, for which he was prescribed a course of ciprofloxacin, and the IPC drain was kept in place. A subsequent dislodgement at home prompted re-evaluation of this strategy given the infected pleural space, the presence of a foreign body, and the patient’s inability to care for the IPC. In order to both achieve local control of the infection and provide durable pleural drainage, an OWT was performed with removal of three adjacent ribs and skin flap creation (Figure 3). The patient went home the first post-operative day without complications and was able to manage his drainage independently at home.

Discussion
The presence of MPE often signifies advanced or metastatic disease and thus is associated with poor prognosis. Prompt evacuation of the pleural cavity is essential for management of pleural infection, and delayed drainage has been associated with higher morbidity (13). There is no consensus regarding the optimal approach to pleural drainage. Thoracocentesis, small and large bore drains, and thoracoscopy all play a potential role (14). Pleuroperitoneal shunting initially represented an attractive approach to drainage of the pleural space, however it is both an invasive procedure and is associated with significant morbidity. As many as half of patients develop shunt occlusion and 33% develop an infection related to the procedure (15). Rare reports of cancer seeding the chest wall (15,16) and pneumoperitoneum (17) have also been reported, thus it has fallen out of favor in the setting of MPE.
Insertion of IPC’s has been shown to be as effective at relieving symptoms of MPE as tube drainage and talc pleurodesis, and it improves quality of life (7). Patients with an IPC perform regular drainages and this may contribute to reducing the bacterial burden and thus the disease severity. IPC has the added benefit of achieving SP in a subset of patients. The recent ASAP trial demonstrated that aggressive drainage on a daily basis as compared to every other day improved the rate of SP to 47% from 24%, with a shorter median time to this endpoint (54 days with daily drainage as compared to 90 days every other day) (18). Initial IPC efficacy, measured objectively as <20% of the hemithorax containing fluid at 2 weeks by chest radiography, also predicts SP (9). Specific patient or cancer related variables, however, have not yet been elucidated in the literature.
Unfortunately, there is an intermediate risk of pleural infection associated with IPC’s. In the TIME2 trial, IPC-related pleural infection was reported in more patients than talc pleurodesis (7). IPC-related pleural infection rate has been reported as high as 5% with a mortality risk of 6% (12). Although Staphylococcus aureus is the most commonly isolated organism, IPC-related pleural infections can arise from a wide range of microbes, thus emphasizing the need for broad-spectrum antibiotic coverage in the initial management of these patients. It is also worth noting that gram-negative infections have been associated with worse outcomes (12). Intravenous antibiotic regimens are favored in this patient population to ensure adequate coverage, especially if methicillin-resistant S. aureus is prevalent in a particular setting. After the patient’s clinical status has been stabilized, the antibiotics can be narrowed and switched to oral regimens for a 3- to 6-week treatment course (14). However, previous observations have supported the role of conservative management of IPC-related infections with oral therapy alone and outpatient follow-up (11,12). Hospitalization, removal of IPC, and IV antibiotics may not be necessary in many patients and ultimately the management should be tailored to disease severity, causative organism, and the individual patient’s infection comorbidity. In the largest cohort of patients with IPC to date, it was found that the majority of pleural infections could be successfully managed without removing the IPC (12). In fact, of the patients in the cohort that had their IPCs removed for infection control, 48% required another IPC or chest tube drainage (12).
When IPC drainage fails, as in our patient, open surgical drainage or OWT, initially introduced by Eloesser in 1935 (19) and subsequently modified by others (20,21), may provide an alternative management approach. OWT both controls the infection and creates a draining fistula. However, this procedure should only be offered to patients that are fit for operative intervention. OWT can be performed either as a definitive treatment with intent to clear the infection, a preliminary procedure prior to definitive treatment, or as a last resort procedure after less invasive approaches have failed. In two large series this technique has been shown to be safe and effective in the setting of chronic pleural infections (21,22). Successful closure of the OWT cavity is achieved in a small percentage of patients. Patients that are medically fit to tolerate another major operation can be offered closure of the cavity at a later date if they have sufficient life expectancy. Ultimately, our patient tolerated OWT well without morbidity and was able to manage pleural cavity drainage independently at home. We thus believe OWT is an excellent alternative approach in selected patients with secondarily infected MPE wherein IPC drainage and antibiotics have failed to clear the infection, the IPC catheter itself fails/is not manageable by the patient, or there is a desire to maintain drainage of the pleural space without the presence of a foreign body.
Acknowledgements
We thank the patient for allowing us to participate in his care and his consent to publish his case for the advancement of research and education.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this Case Report and any accompanying images.
References
- American Thoracic Society. Management of malignant pleural effusions. Am J Respir Crit Care Med 2000;162:1987-2001. [Crossref] [PubMed]
- Antunes G, Neville E, Duffy J, et al. BTS guidelines for the management of malignant pleural effusions. Thorax 2003;58:ii29-38. [Crossref] [PubMed]
- Lombardi G, Zustovich F, Nicoletto M, et al. Diagnosis and treatment of malignant pleural effusion: a systematic literature review and new approaches. Am J Clin Oncol 2010;33:420-3. [Crossref] [PubMed]
- Penz E, Watt KN, Hergott CA, et al. Management of malignant pleural effusion: challenges and solutions. Cancer Manag Res 2017;9:229-41. [Crossref] [PubMed]
- Van Meter ME, McKee KY, Kohlwes RJ. Efficacy and safety of tunneled pleural catheters in adults with malignant pleural effusions: a systematic review. J Gen Intern Med 2011;26:70-6. [Crossref] [PubMed]
- Gilbert CR, Lee HJ, Skalski JH, et al. The use of indwelling tunneled pleural catheters for recurrent pleural effusions in patients with hematologic malignancies. Chest 2015;148:752-8. [Crossref] [PubMed]
- Davies HE, Mishra E, Kahan B, et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA 2012;307:2383-9. [Crossref] [PubMed]
- Sioris T, Sihvo E, Salo J, et al. Long-term indwelling pleural catheter (PleurX) for malignant pleural effusion unsuitable for talc pleurodesis. Eur J Surg Oncol 2009;35:546-51. [Crossref] [PubMed]
- Tremblay A, Michaud G. Single-center experience with 250 tunneled pleural catheter insertions for malignant pleural effusion. Chest 2006;129:362-8. [Crossref] [PubMed]
- Suzuki K, Servais E, Rizk NP, et al. Palliation and pleurodesis in malignant pleural effusion: the role for tunneled pleural catheters. J Thorac Oncol 2011;6:762-7. [Crossref] [PubMed]
- Bibby AC, Clive A, Slade G, et al. Survival in patients with malignant pleural effusions who developed pleural infections. Chest 2015;148:235-41. [Crossref] [PubMed]
- Fysh ET, Tremblay A, Feller-Kopman D, et al. Clinical outcomes of indwelling pleural catheter-related pleural infections: an international multicenter study. Chest 2013;144:1597-602. [Crossref] [PubMed]
- Tobin CL, Lee YG. Pleural infection: what we need to know but don’t. Curr Opin Pulm Med 2012;18:321-5. [Crossref] [PubMed]
- Tremblay A, Stather DR, Maceachern P. How should we man- age empyema complicating tunneled pleural catheter place- ment? J Bronchology Interv Pulmonol 2010;17:106-8. [Crossref] [PubMed]
- Genc O, Petrou M, Ladas G, et al. The long-term morbidity of pleuroperitoneal shunts in the management of recurrent malignant effusions. Eur J Cardiothorac Surg 2000;18:143-6. [Crossref] [PubMed]
- Baeyens I, Berrisford RG. Pleuroperitoneal shunts and tumor seeding. J Thorac Cardiovasc Surg 2001;121:813. [Crossref] [PubMed]
- Beshay M, Schmid RA. Spontaneous pneumoperitoneum after pleuroperitoneal shunt. Eur J Cardiothorac Surg 2003;23:239. [Crossref] [PubMed]
- Wahidi MM, Chakravarthy R, Yamus L, et al. Randomized control trial of pleural drainage frequency in patients with malignant pleural effusions: the ASAP trial. Am J Respir Crit Care Med 2017;195:1050-7. [Crossref] [PubMed]
- Eloesser L. Of an operation for tuberculous empyema. Ann Thorac Surg 1969;8:355-7. [Crossref] [PubMed]
- Symbas PN, Nugent JT, Abbott OA, et al. Nontuberculous pleural empyema in adults: the role of a modified Eloesser procedure in its management. Ann Thorac Surg 1971;12:69-78. [Crossref] [PubMed]
- Thourani VH, Lancaster RT, Mansour KA, et al. Twenty-six years of experience with the modified Eloesser flap. Ann Thorac Surg 2003;76:401-5. [Crossref] [PubMed]
- Reyes KG, Mason DP, Murthy SC, et al. Open window thoracostomy: modern update of an ancient operation. Thorac Cardiovasc Surg 2010;58:220-4. [Crossref] [PubMed]
Cite this article as: Villano AM, Caso R, Marshall MB. Open window thoracostomy as an alternative approach to secondarily infected malignant pleural effusion and failure of intrapleural catheter drainage: a case report. AME Case Rep 2018;2:12.


