An anaplastic lymphoma kinase (ALK) fusion oncogene positive metastatic sarcomatoid carcinoma of the lung with good response to crizotinib
Introduction
Primary sarcomatoid carcinoma (SC) of the lung is a rare and highly malignant tumor and accounts for less than 1% of all lung cancers (1-3). As recently established by the World Health Organization (WHO), the pulmonary SC is classified into carcinosarcoma, pleomorphic carcinoma, spindle cell carcinoma, giant cell carcinoma and pulmonary blastoma (4).
Pulmonary SC more commonly affects men with a history of smoking and has also been described with asbestosis (5-7). The average age of diagnosis is 60 years and there is 4 times greater preponderance in men (8,9).
As long as the tumor is operable, surgery is the treatment of choice. Postsurgical radiotherapy can be well applied, especially when the resection is incomplete (10). For metastatic disease there is currently no data available and patients are usually treated with the same cytotoxic agents as non-small cell lung cancers (NSCLC), but in most cases, chemoresistance appears and this might be the reason for poor prognosis (11,12).
During the last decade, there has been an immense development of targeted and immunotherapy in the lung cancer area, which has improved the survival outcomes. Although epithermal growth factor receptor (EGFR)-, anaplastic lymphoma kinase (ALK)-, programmed death-1 (PD-1)- and programmed death-ligand 1 (PD-L1)-targeted therapies have a promising therapeutic effect in patients with typical NSCLC, especially such as adenocarcinoma, the potential clinical effect of these drugs to SC is still unknown (13-15). Therefore, we report a case of carcinosarcoma with ALK fusion gene with good response to therapy with crizotinib.
Case presentation
A 50-year-old male was hospitalized due to right pleural effusion for further investigation. He had complained for 2 months of progressive non-productive cough, fatigue and light pain in the right axillary region. The patient had a smoking history of 7 pack years, he had quit 5 years ago. Upon physical examination, the patient was in a stable condition, ECOG 1, with no concomitant pathologies. Lung auscultation revealed the absence of respiratory sounds in the right lower lobe.
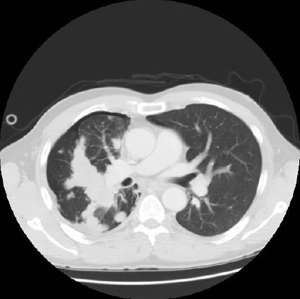
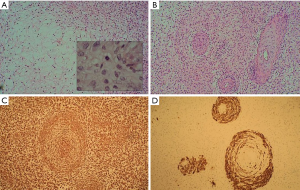
A CT scan was performed which showed a central tumor with multiple different sized metastases in the thorax and right pleural effusion (Figure 1). A chest tube instantly removed 1 liter of haemorrhagic liquid, cytologically full of atypical cells. Endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) found atypical cells, which were immunohistochemically characteristic to adenocarcinoma. For more accurate diagnosis biopsies were taken from the right parietal pleura. Pathohistology revealed mesenchymal tissue with spindle cells and many vascular structures (Figure 2A). Also, areas with epithelial component were seen (Figure 2B). Tumor cells were positive for vimentin (Figure 2C) and pan-keratin (Figure 2D). Histologically and immunohistochemically the pattern was primarily suggestive for carcinosarcoma. As the epithelial cells were positive for p40 and CK5/6, the epithelial component was more characteristic of a squamous cell carcinoma.
In the multidisciplinary tumor board, a palliative systemic chemotherapy was recommended and the patient received one cycle with cisplatin and docetaxel. During the 1st cycle of chemotherapy, a broad molecular profiling of the tumor tissue was performed (OncoDeepDx+ test, OncoDNA SA, Belgium). Molecular profiling showed no mutations in the common oncogenes, including EGFR, BRAF, ERBB2, MET, MEK, RAS; PD-L1 had a low expression, but the presence of ALK-EML4 fusion was diagnosed. Therefore, from February 9, 2016 the therapy was switched to ALK inhibitor crizotinib 250 mg twice daily.
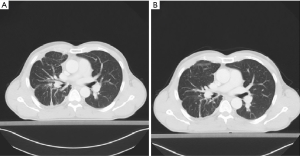
The patient has tolerated the treatment well, except for the mild augmentation of liver enzymes and the development of a light cough (grade 1) at the beginning of the therapy, which disappeared. Tumor changes are characterized in Figure 3. The patient has now more than a year a partial response and good tolerability to crizotinib treatment. Currently, the patient’s ECOG status is 0 and the therapy with crizotinib continues.
Discussion
We report a case of an ALK-positive carcinosarcoma with a good response to therapy with crizotinib. Crizotinib is effective in ALK-positive NSCLC (16), but in the case of carcinosarcomas, such data is missing or limited.
Primary SC of the lung is a rare and highly malignant tumor and accounts for less than 1% of all lung cancers (1-3). There is no good standard therapy available for the cure of metastatic SC, patients are currently treated as NSCLC with similar chemotherapy (15). Many recent case reports and studies, which have focused on SC, have shown worse survival and outcome compared to other histological subtypes of NSCLC (17). Therefore, we can conclude that the standard treatment of NSCLC is not as effective among SC patients.
During the last decade there has been a significant development in the field of targeted therapies and immunotherapy of NSCLC with the routine use of EGFR and ALK inhibitors and more recently also PD-1 inhibitors. Guidelines from the College of American Pathologists (CAP), the International Association for the Study of Lung Cancer (IASLC), and the Association of Molecular Pathologists (AMP) recommend the analysis of either the primary tumor or of a metastasis for EGFR and ALK for all patients whose tumor contains an element of adenocarcinoma, regardless of the clinical characteristics of the patient (18,19), but the approach in SC is less clear.
Targetable genetic aberrations in NSCLC have also been investigated in SC.
One of these targets is EGFR (20). A study by Italiano et al. analyzed EGFR and KRAS mutational status among 22 cases of primary lung SC, where KRAS mutation was found in eight cases, no EGFR mutation was detected (13). Also, Terra et al. analyzed molecular characterizations of lung SC in 33 cases and didn’t find EGFR mutations, but in 20% of cases a KRAS mutation was detected (14). These findings may suggest that the overexpression of EGFR protein and high rate of KRAS mutation could be the reason for poor prognosis compared to other types of NCSLC.
SC may benefit from therapies targeting PD-1 protein and PD-L1 (21). A study by Velcheti et al. found that SC have higher PD-L1 levels than NSCLC. They analyzed two large retrospective lung cancer cohorts, where 9 of 13 patients with SC were positive for PD-L1. This study supports the potential clinical effect of using anti-PD-1/PD-L1 targeted therapies in the treatment of metastatic SC (15). Our patient had low expression of PD-L1.
In a recent study by Terra et al. (14), they analyzed almost 2,800 mutations in 33 cases of lung SC by next-generation sequencing. Twenty-four of 33 cases had at least 1 abnormality. The most common were TP53 mutations (19 cases), which were followed by KRAS mutations (10 cases), AKT1, JAK3, BRAF, NRAS and PIK3CA mutations (1 case in each), no EGFR mutations were found. ALK rearrangement, which has been a rare finding among SC so far, occurred in one case and currently we have potential targeted treatment for this mutation. However, a study by Chen et al. showed that the incidence of ALK rearrangement (5/141, 3.5%) in SC is similar to other subtypes of NSCLC and more often among young patients with no smoking history (22).
Those studies show the importance of testing SC also for targetable mutations to find potential therapeutic options (14). Chen et al. have reported a case of ALK-positive pleomorphic carcinoma with partial response to crizotinib (23). Our patient with metastatic carcinosarcoma had ALK rearrangement and is currently receiving crizotinib with good response and has stable disease for more than 1 year.
Conclusions
Pulmonary SC is a rare and aggressive tumor, which is partly pathomorphologically and molecularly different from other types of NSCLC. In metastatic disease, chemoresistance appears fast and so far, there is no comprehensive data about potential targeted therapies in SC. An ALK rearrangement is a rare finding in SC, but as the current clinical case demonstrates, it may occur and with currently available treatment with crizotinib there might be a good response and a better outcome. SC should be evaluated for potentially targetable mutations in the same manner as other NSCLC, especially adenocarcinoma. Broad molecular profiling or at least EGFR mutation, ALK, ROS1 and PD-L1 testing should be a standard of care among SC patients to better understand molecular characteristics and to find a possible treatment.
Acknowledgements
None
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Davis MP, Eagan RT, Weiland LH, et al. Carcinosarcoma of the lung: Mayo Clinic experience and response to chemotherapy. Mayo Clin Proc 1984;59:598-603. [Crossref] [PubMed]
- Huwer H, Kalweit G, Straub U, et al. Pulmonary carcinosarcoma: diagnostic problems and determinants of the prognosis. Eur J Cardiothorac Surg 1996;10:403-7. [Crossref] [PubMed]
- Hountis P, Moraitis S, Dedeilias P, et al. Sarcomatoid lung carcinomas: a case series. Cases J 2009;2:7900. [Crossref] [PubMed]
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors. Available online: http://www.jto.org/article/S1556-0864(15)33571-1/pdf
- Pelosi G, Sonzogni A, De Pas T, et al. Review article: pulmonary sarcomatoid carcinomas: a practical overview. Int J Surg Pathol 2010;18:103-20. [Crossref] [PubMed]
- Nakajima M, Kasai T, Hashimoto H, et al. Sarcomatoid carcinoma of the lung: a clinicopathologic study of 37 cases. Cancer 1999;86:608-16. [Crossref] [PubMed]
- Humphrey PA, Scroggs MW, Roggli VL, et al. Pulmonary carcinomas with a sarcomatoid element: an immunocytochemical and ultrastructural analysis. Hum Pathol 1988;19:155-65. [Crossref] [PubMed]
- Ishida T, Tateishi M, Kaneko S, et al. Carcinosarcoma and spindle cell carcinoma of the lung. Clinicopathologic and immunohistochemical studies. J Thorac Cardiovasc Surg 1990;100:844-52. [PubMed]
- Koss MN, Hochholzer L, Frommelt RA. Carcinosarcomas of the lung: a clinicopathologic study of 66 patients. Am J Surg Pathol 1999;23:1514-26. [Crossref] [PubMed]
- Braham E, Ben Rejeb H, Aouadi S, et al. Pulmonary carcinosarcoma with heterologous component: report of two cases with literature review. Ann Transl Med 2014;2:41. [PubMed]
- Mason RJ, Broaddus VC, Martin T, et al. Murray and Nadel's Textbook of Respiratory Medicine. 5th edition. Rare malignant primary pulmonary epithelial tumors. Ch. 48, Part 4. Primary Pulmonary Sarcomas. 2010.
- Ouziane I, Boutayeb S, Mrabti H, et al. Sarcomatoid carcinoma of the lung: a model of resistance of chemotherapy. N Am J Med Sci 2014;6:342-5. [Crossref] [PubMed]
- Italiano A, Cortot AB, Ilie M, et al. EGFR and KRAS status of primary sarcomatoid carcinomas of the lung: implications for anti-EGFR treatment of a rare lung malignancy. Int J Cancer 2009;125:2479-82. [Crossref] [PubMed]
- Terra SB, Jang JS, Bi L, et al. Molecular characterization of pulmonary sarcomatoid carcinoma: analysis of 33 cases. Mod Pathol 2016;29:824-31. [Crossref] [PubMed]
- Velcheti V, Rimm DL, Schalper KA. Sarcomatoid lung carcinomas show high levels of programmed death ligand-1 (PD-L1). J Thorac Oncol 2013;8:803-5. [Crossref] [PubMed]
- Solomon BJ, Mok T, Kim DW, et al. First-Line Crizotinib versus Chemotherapy in ALK-Positive Lung Cancer. N Engl J Med 2014;371:2167-77. [Crossref] [PubMed]
- Martin LW, Correa AM, Ordonez NG, et al. Sarcomatoid carcinoma of the lung: a predictor of poor prognosis. Ann Thorac Surg 2007;84:973-80. [Crossref] [PubMed]
- Lindeman NI, Cagle PT, Beasley MB, et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. Arch Pathol Lab Med 2013;137:828-60. [Crossref] [PubMed]
- Leighl NB, Rekhtman N, Biermann WA, et al. Molecular testing for selection of patients with lung cancer for epidermal growth factor receptor and anaplastic lymphoma kinase tyrosine kinase inhibitors: American Society of Clinical Oncology endorsement of the College of American Pathologists/International Association for the study of lung cancer/association for molecular pathology guideline. J Clin Oncol 2014;32:3673-9. [Crossref] [PubMed]
- Herbst RS. Review of epidermal growth factor receptor biology. Int J Radiat Oncol Biol Phys 2004;59:21-6. [Crossref] [PubMed]
- Velcheti V, Schalper KA, Carvajal DE, et al. Programmed death ligand-1 expression in non-small cell lung cancer. Lab Invest 2014;94:107-16. [Crossref] [PubMed]
- Chen X, Zhang Y, Lu J, et al. Pulmonary Sarcomatoid Carcinoma with ALK Rearrangement: Frequency, Clinical-Pathologic Characteristics, and Response to ALK Inhibitor. Transl Oncol 2017;10:115-20. [Crossref] [PubMed]
- Chen X, Liang J, Lu J, et al. Pulmonary Sarcomatoid Carcinoma with ALK rearrangement: Frequency, Clinical-Pathological Characteristics, and Response to ALK inhibitor. J Clin Oncol 2016;34:15_suppl:e20055.
Cite this article as: Valter A, Roosipuu R, Tamm H, Padrik P. An anaplastic lymphoma kinase (ALK) fusion oncogene positive metastatic sarcomatoid carcinoma of the lung with good response to crizotinib. AME Case Rep 2018;2:2.


