Enigma of recurrent strokes with literature review
Introduction
Cardiac fibroelastoma is a rare primary cardiac tumor, typically found on autopsy or resections. As advancement of diagnostic modalities in cardiology, the reporting and diagnosis of these tumors have been more sporadic with majority as case reports. Tumors are described as small, well-defined pedunculated mass with a predilection of valvular endocardium. Though echocardiography has improved the diagnosis, it still remains uncertain to diagnose between cardiac myxomas and fibroelastoma, as it requires pathological determination for the cases of fibroelastoma. Here we discuss a case about a 21 years old male with history of recurrent strokes on anticoagulation diagnosed with cardiac papillary fibroelastoma (CPF) upon resection.
Case presentation
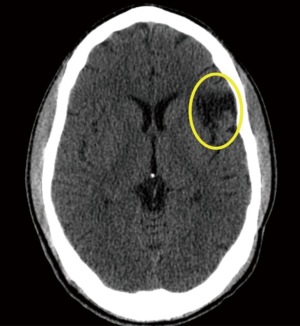
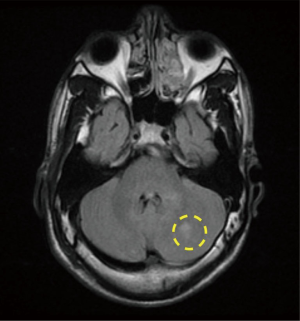
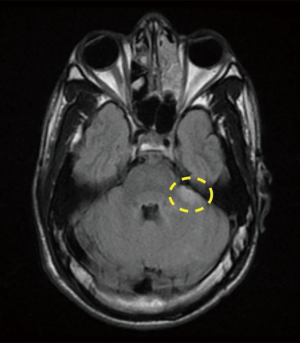
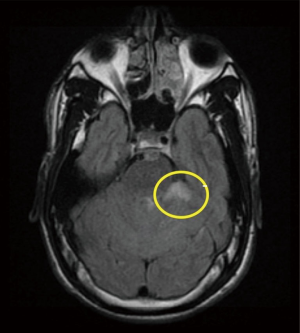
A 21-year-old African American male with the history of hypertension presenting with 6 hours history of right lower leg weakness and numbness with intractable nausea, vomiting and photophobia. He denied any chest pain, shortness of breath, syncope, dysphagia, or any other focal neurological deficit. There was no history of head and neck trauma. Two years prior to admission, he experienced left frontal and parietal lobe ischemic stroke (seen on previous CAT scan) for which he was maintained on aspirin and high intensity statins (Figure 1). Physical examination showed blood pressure 160/84 mmHg, heart rate 75 beats/min, respiratory rate 20 breaths/min. Patient had vertical nystagmus of the right eye, loss of fine and deep touch of right lower limb with muscle strength of 4/5, dysmetria and dysdiadochokinesis were noted on left side. Since the patient was out of the window period no TPA was given. Brain magnetic resonance imaging (MRI) showed multiple tiny infarcts in left cerebellar hemisphere and old left frontoparietal infarct (Figures 2–4).
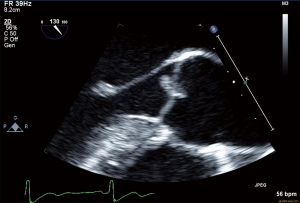
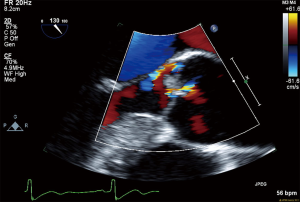
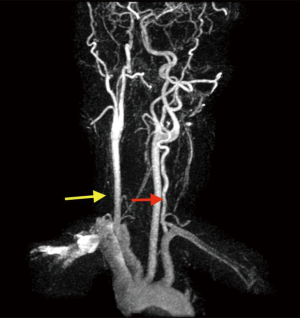
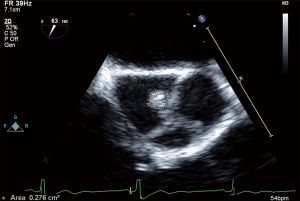
All routine blood work performed including lipid panel, TSH, B-12 level and urine drug screen were within normal limits. Transthoracic echocardiogram (TTE) showed an EF of 55% with no other intra-cardiac masses/vegetations or clots seen. In regard of recurrent ischemic stroke in a young patient work-up for underlying thrombophilia and vasculitis was within normal limits. The possibility of underlying embolic etiology of stroke was our major concern whether it was cardiac or extra cardiac, therefore magnetic resonance angiogram (MRA) neck was ordered showing absent flow of right vertebral artery and tortuous left vertebral artery, which was highly suggestive of embolic phenomenon (Figure 5). Cerebral angiogram showed there is no contribution of intracranial flow from right vertebral artery and there is right retrograde flow in the distal right vertebral artery through left vertebral injection, the distal right vertebral artery is irregular and stenotic and leans to flow within the right posterior inferior cerebellar artery (PICA) distribution, no evidence of aneurysm, dissection or arteriovenous malformation (AVM). Transesophageal echocardiography (TEE) noted multiple echogenic masses on the right coronary and the noncoronary cusp with mild aortic regurgitation (Figures 6,7,8). In the presence of aortic valve masses suggestive etiology cause of cardio-embolic phenomenon in this young patient cardiac surgery was called, and surgical excision of the aortic valve with AVR using metallic prosthesis regarding his young age was done. The patient had a smooth post-operative course. Pathology of the aortic masses showed myxoid degeneration and fibrinous exudates that is consistent with papillary fibroelastoma (PFE).
Discussion
Primary cardiac tumors are rare entity that usually diagnosed on the autopsy or incidental findings for some other diagnosis. A study reported seven tumors for over 12,000 autopsies, while Wang et al. reported incidence of primary tumor as 0.056% and for secondary heart tumors as 1.23% for 12,485 autopsies (1,2). CPF is described consisting a papillary structure arising from any endocardial surface with predilection for cardiac valves, especially aortic or mitral valves (3). The left ventricle was the predominant nonvalvular site involved. The majority of clinical observation and data for fibroelastoma reported from the retrospective studies. There has no clear risk factor identified for fibroelastoma. The size of the tumor varied from 2 to 70 mm. It usually presented with transient ischemic attack (TIA), cerebrovascular accident (CVA), myocardial infarction (MI), sudden death, heart failure, presyncope, syncope, pulmonary embolism (PE), blindness, and peripheral embolism. Tumor mobility was the only independent predictor of PFE-related death or nonfatal embolization.
Primary cardiac tumors can manifest symptoms via several different mechanisms: (I) obstruction of intracardiac flow causing valvular dysfunctions, mitral valve regurgitation and congestive heart failure (CHF); (II) invasion of cardiac tissue can lead to arrhythmia and pericardial effusion and tamponade; (III) detachment of tumor and embolization; and (IV) tumors may produce constitutional symptoms (4).
Right heart cardiac tumors often presented with outlet obstruction, tricuspid or pulmonic stenosis, and right heart failure, such as fatigue, peripheral edema, hepatomegaly, ascites, and prominent “a” waves along with diastolic murmur. In addition, these tumors release fragments into the pulmonary circulation, causing symptoms consistent with pulmonary emboli. Patients with patent foramen ovale may present with right atrial hypertension, resulting from shunting of venous blood into the systemic circulation causing hypoxemia or systemic emboli (5).
While patient with left heart cardiac tumors can present with mitral or aortic valvular dysfunctions, left heart failure, secondary pulmonary hypertension with symptoms of dyspnea orthopnea, paroxysmal nocturnal dyspnea, pulmonary edema, cough, hemoptysis, edema, and fatigue. Symptoms may be worse in certain body positions due to motion of the tumor within the atrium. On physical examination, a characteristic “tumor plop” may be heard early in diastole (5,6). In a case series study of 74 patients with atrial myxomas, Auger et al. reported about 12% of patient presenting with neurological sequelae on admission that were related systemic myxomatous tumor embolization. Left ventricular tumors can arise intra-cavitary or may invade myocardium causing outflow obstruction that may lead to systemic embolization, arrhythmias or conduction defects leading to patients presenting with syncope or systolic heart failure (5–7). Fibroelastoma arising from aortic valve often lead to ischemia or ventricular fibrillation causing sudden cardiac death, which had previously published in many case reports (8). There have been few cases of fibroelastoma presenting in extra-cardiac locations, such as infiltrating tissue and mimicking bronchogenic carcinoma (9). Fibroelastoma may have constitutional symptoms of fever, night sweats, weight loss and laboratory abnormalities that suggest the presence of connective tissue diseases. Although the etiology of these symptoms is not fully understood, the production of various cytokines and growth factors by the tumor may contribute to these clinical and laboratory abnormalities. Constitutional symptoms were seen in 34% of patients. Laboratory abnormalities (e.g., anemia and elevations in the erythrocyte sedimentation rate, C-reactive protein, or globulin level) were present in 37%, usually those with systemic symptoms (10).
Echocardiogram remains preferred choice of diagnostic modality for presumptive diagnosis. It helps detect tumor, precise location and defines the extent along with prediction of the tumor type (5). In a retrospective review of cardiac fibroelastoma, Kassop et al. reported detection of fibroelastoma in 141 patients on echocardiography, while 48 patients had PFE’s that were not visible by echocardiography, but found to have tumor size 2,6). TEE remains superior to TTE for primary cardiac tumor because close proximity between esophagus and heart and lack of interfering lung tissues, bony tissues and also TEE has high-frequency imaging transducers for greater resolution (9). Cardiac MRI and cardiac computed tomography (CT) may be another noninvasive testing for the diagnosis of primary cardiac tumors (10). Surgical resection along with pathological evaluation remains the gold standard for the diagnosis and strongly recommended for symptomatic patients to prevent further cardiovascular or embolic events (11).
Symptomatic patients should be treated surgically because the successful complete resection of PFE is curative and the long-term postoperative prognosis is excellent. Given the potential to cause fatal outcomes and availability of therapies, surgery should be strongly considered for: (I) history of embolic events or at risk of embolization; (II) coronary artery disease secondary to the mass; (III) mobile fibroelastoma; and (IV) size of tumor >1 cm (12).
The symptomatic patients who are not surgical candidates could be offered long-term oral anticoagulation, although no randomized controlled data is available on its efficacy. Asymptomatic patients could be treated surgically if the tumor is mobile, as the tumor mobility is the independent predictor of death or nonfatal embolization. Asymptomatic patients with a non-mobile PFE could be followed-up closely with periodic clinical evaluation and echocardiography, and receive surgical intervention when symptoms develop or the tumor becomes mobile (5). Recurrence of PFE following surgical resection has not been reported.
Conclusions
Primary cardiac tumors are rare with PFE being the second most common after myxoma. In patients with presumed cardio-embolic etiology of stroke cardiac imaging should be offered to rule out underlying cardio-embolic source starting with TTE and proceeding to TEE especially when cardiac masses/tumors is of a concern. Once a presumptive diagnosis of primary cardiac tumor (PFE) has been made on imaging studies, surgical resection should be next especially in symptomatic patients to abolish further embolic risk.
Acknowledgements
We would like to thank Dr. Sundermurthy Yamini, MD and Dr. Fayez Shamoon, MD for providing guidance during the preparation of this case report.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this Case Report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
References
- Wang Z, Chen S, Zhu M, et al. Risk prediction for emboli and recurrence of primary cardiac myxomas after resection. J Cardiothorac Surg 2016;11:22. [Crossref] [PubMed]
- Kassop D, Donovan MS, Cheezum MK, et al. Cardiac Masses on Cardiac CT: A Review. Curr Cardiovasc Imaging Rep 2014;7:9281. [Crossref] [PubMed]
- Munhoz Da Fontoura Tavares C, Araújo De Oliveira N, Miguel R, et al. Recurrent ventricular fibrillation secondary to aortic valve tumor. Heart Rhythm 2004;1:348-51. [Crossref] [PubMed]
- Reynen K. Frequency of primary tumors of the heart. Am J Cardiol 1996;77:107. [Crossref] [PubMed]
- Vander Salm TJ. Unusual primary tumors of the heart. Semin Thorac Cardiovasc Surg 2000;12:89-100. [Crossref] [PubMed]
- Auger D, Pressacco J, Marcotte F, et al. Cardiac masses: an integrative approach using echocardiography and other imaging modalities. Heart 2011;97:1101-9. [Crossref] [PubMed]
- Sheu CC, Lin SF, Chiu CC, et al. Left atrial sarcoma mimicking obstructive pulmonary disease. J Clin Oncol 2007;25:1277-9. [Crossref] [PubMed]
- Jain D, Maleszewski JJ, Halushka MK. Benign cardiac tumors and tumor like conditions. Ann Diagn Pathol 2010;14:215-30. [Crossref] [PubMed]
- ElBardissi AW, Dearani JA, Daly RC, et al. Embolic potential of cardiac tumors and outcome after resection: a case-control study. Stroke 2009;40:156-62. [Crossref] [PubMed]
- Engberding R, Daniel WG, Erbel R, et al. Diagnosis of heart tumours by transoesophageal echocardiography: a multicentre study in 154 patients. European Cooperative Study Group. Eur Heart J 1993;14:1223-8. [Crossref] [PubMed]
- Grandmougin D, Fayad G, Moukassa D, et al. Cardiac valve papillary fibroelastomas: clinical, histological and immunohistochemical studies and a physiopathogenic hypothesis. J Heart Valve Dis 2000;9:832-41. [PubMed]
- Kuon E, Kreplin M, Weiss W, et al. The challenge presented by right atrial myxoma. Herz 2004;29:702-9. [Crossref] [PubMed]
Cite this article as: Kumar V, Nanavati SM, Abuarqoub A, Rushdy A, Rahman M, Komal F, Michael P. Enigma of recurrent strokes with literature review. AME Case Reports 2017;1:5.